Understanding Global Regulatory Pathways For Clinical Development Strategy

Learning about global regulatory requirements and understanding the pathway for approval can help clinical development teams take their global strategy in the right direction. Sharing expertise from regulatory experts for those who need it. (This article was originally published on LinkedIn).

It’s no news that we are now living in an era with a tremendous surge of new therapy developments worldwide. This includes the development of new drugs, biological therapies, and medical devices.
However, when one focuses on clinical development across the globe, it’s hard to ignore the concentration of clinical trials in a couple of geographic regions. The US and Western Europe, predominantly. (See the worldwide distribution of all clinical studies on clinicaltrials.gov)
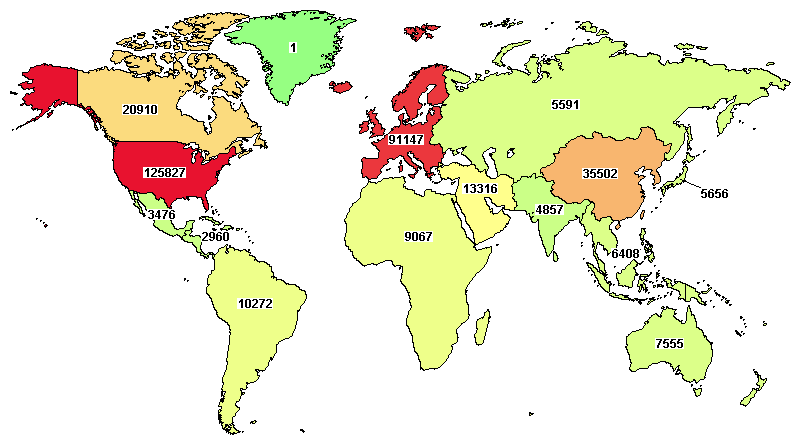
Among several reasons for this concentration, clarity on regulatory requirements, and awareness of a faster approval process stand as ‘external’ factors, consistently being managed with a deeper understanding to steer through. Other factors, such as availability of patients, trained & experienced researchers, infrastructure, etc, are managed differently or considered ‘unmanageable’.
Nevertheless, more and more development programs are now considering regions beyond the US and Western Europe. For instance, Clinicaltrials.gov shows an interesting comparison of active clinical trials versus total clinical trials conducted in the US and China. The US has 36% of active studies globally now, while overall 39% of all clinical trials have been conducted there. However, China has 14% active clinical trials now compared to 11% of the total studies conducted globally.
(See the worldwide distribution of recruiting / not yet recruiting clinical studies on clinicaltrials.gov)
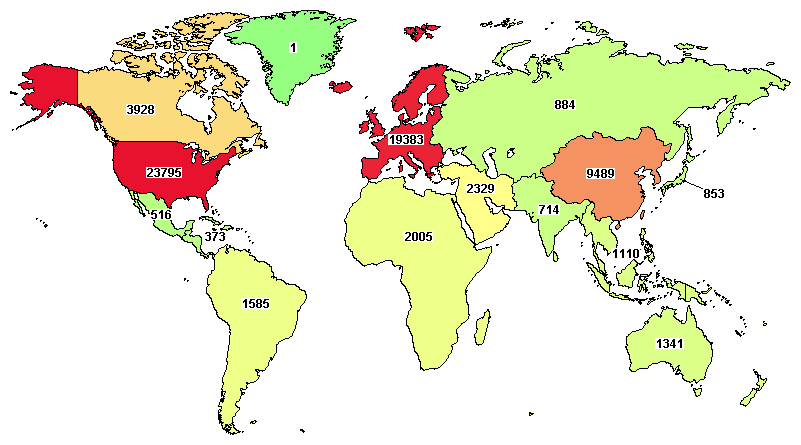
The message is clear. With a greater understanding of regulatory processes and market requirements, researchers are finding it easier to focus on new regions, such as China or East Asia.
Other regions, such as Eastern Europe, South East Asia, Pacific region, and Latin America are also showing considerable promises by improvising and clarifying their regulatory approval process for initiating clinical trials.
Learning about these processes and understanding the pathway for approval can help clinical development teams develop their global strategy in the right direction.
1. East Asia
- China:
- Taiwan:
- South Korea:
- Japan:
- Hong Kong:
2. Southeast Asia
- Thailand:
- Thailand’s Clinical Trial Regulatory Scenario – Simplified (1/2),
- Thailand’s Clinical Trial Regulatory Scenario – Simplified (2/2),
- UPDATE 1: Thailand’s Clinical Trial Regulatory Scenario,
- UPDATE 2: Thailand’s Latest Approved IRB List,
- Marketing Authorization & Regulatory Requirements for Drug Registration in Thailand
- Vietnam:
- Philippines:
- Malaysia:
- Singapore:
- Indonesia:
3. South Asia
- India:
- Sri Lanka:
4. Eastern Europe
- Romania:
- Poland:
- Ukraine:
- Russia:
5. Pacific Region
- Australia:
- New Zealand:
6. Latin America
At Credevo, we have brought together regulatory experts, who are highly experienced in dealing with approval processes for their respective agencies. These experts have also proven their capabilities time and again by ensuring smooth processing of regulatory applications for our clients.
We invite you to discuss with these regulatory experts about your specific regulatory needs.
Contact Credevo or just send us a message through social media or other means (links are given below).
Kshitij
Contact Credevo team via Credevo.com
Explore Credevo’s works and free knowledge support through blogs
Contact or follow Credevo on social media: LinkedIn, Twitter, Facebook
Contact or follow Kshitij on LinkedIn