Why Your Clinical Trials Should Include Sites in Romania?

The best reasons to conduct clinical trials in any country include the availability of patients, clarity of regulatory processes, willing and qualified investigators, and sound infrastructure at a reasonable cost. This is what you get if you conduct clinical trials in Romania. Let’s see this in greater detail.

Brief demography
- Romania is a sovereign state located in Southeastern Europe near Bulgaria, Ukraine, Hungary, Serbia, and Moldova. It is a developing country, part of the European Union, and is rapidly growing.
- Romania has an economy predominantly based on services and is cementing its reputation as an industrious, educated, and ambitious region in many sectors.
- Romania has one of the larger populations in the region with over 22 million. About 55% of the population resides in the urban areas with major concentrations in the cities of Iasi, Timisoara, Constanta, Cluj-Napoca, and Craiova.
Top 7 advantages of conducting clinical trials in Romania
The increased interest in Romania for clinical trials is not without sound reasons. Several factors provide great advantages to Romania in conducting clinical trials.
- Recruitment
- High patient availability
- Faster recruitment timelines
- Expertise
- High standards of medical education,
- Highly concentrated and specialized healthcare services,
- Experienced personnel (including eCRF and IVRS solutions),
- Relevant expertise
- Infrastructure
- National infrastructure and environment,
- Supportive infrastructure for clinical trials
- Cost
- Cost-efficiency in operations
- Great competitive cost per patient
- Quality
- Good regulatory and protocol compliance with good-quality data
- Sites certified by audits and inspections conducted by sponsor, regulatory or third parties.
- Time
- Trial start-up times and requirements which are comparable with other EU countries,
- Clarity about regulatory timelines
- Regulatory
- Unambiguous regulatory conditions,
- Synchronization with EU regulations
- Almost all European guidelines and rules governing medicinal products in the EU have been transposed into national legislation in Romania
Clinical trials status
Romania has a decent number of clinical trials to its share. The number of clinical trials is growing after economic changes in the late 20 century.
- Total number of studies in Romania – 2,618 (Clinicaltrials.gov)
- Total number of recruiting studies in Romania – 265 (Clinicaltrials.gov)
Several clinical research organizations now include Romania as their region of work. Most of these are international, if not global, CROs and serve to connect Romania with other regions of clinical research.
Regulatory authority (RA)
National Agency for Medicines and Medical Devices (NAMMD) serves as the regulatory authority governing the authorization of clinical trials in Romania.
NAMMD grants clinical trial authorization to the sponsor or to the contracting research organization (CRO) acting on behalf of the sponsor.
In some exceptions, in investigator-driven studies, the authorization can be granted to the investigator(s) initiating the trial, who will have certain attributions similar to a sponsor.
The regulatory approval process
The regulatory process for clinical trials approval in Romania is transparent and relatively fast.
Let’s discuss the regulatory process under three different kinds of approvals.
- Regulatory Authority (RA)
- Ethics Committee (IRB)
- Import Licence
Ethics Committee (IRB)
- According to Ministry of Health Order 904/2006 on the implementation of good practices in the performance of clinical studies, clinical trial activities are not permitted without a favorable opinion from the National Agency for Medicines and Medical Devices’ ethics committee.
- This ethics body (National Ethics Commission) serves as the IRB arm for approval of clinical trials.
Import License
Import approval of the study drug is no longer necessary since the drugs are being imported based on the study approval.
Process of submission to RA & IRB
- The submission of the study documents can be done in parallel to both the competent regulatory authority (the National Medicines Agency) and the ethics body (National Ethics Commission), or the applicant may request an IRB opinion before filing an authorization request..
- According to National Agency for Medicines and Medical Devices Scientific Council Decision 2/2014, clinical studies must be performed in authorized units only.
- The unit authorization is valid for two years and may be extended upon request.
- The scope of the authorization, as well as the necessary documentation, differs according to the type of trial the solicitant wishes to conduct (eg, authorization for clinical studies with therapeutic benefits, authorization for Phase I clinical trials, and authorization for bioequivalence clinical trials).
- Applicant must notify RA by way of a signed letter of intent concerning the payment of the authorization fee and its intention to apply at least two weeks in advance.
- Applicant must submit the documents listed in Annex 1 to Decision 6/2014 in electronic form. These documents include a request for authorization, opinion of the ethics committee, information about the sponsor, and the medicine file.
- It’s strongly recommended for applicants not to bring any important amendments to the initial request after 50 days following the payment of the authorization fee.
- The signed approval order indicates the institution where the study will be conducted, and the principal investigator responsible for conducting it.
- The authorization for clinical studies is valid for the period of the study. However, it loses validity if the study does not begin within one year of authorization being issued.
- RA needs to be informed about the study’s start date and any amendments to the study plan. Applicants must also notify RA when the study concludes.
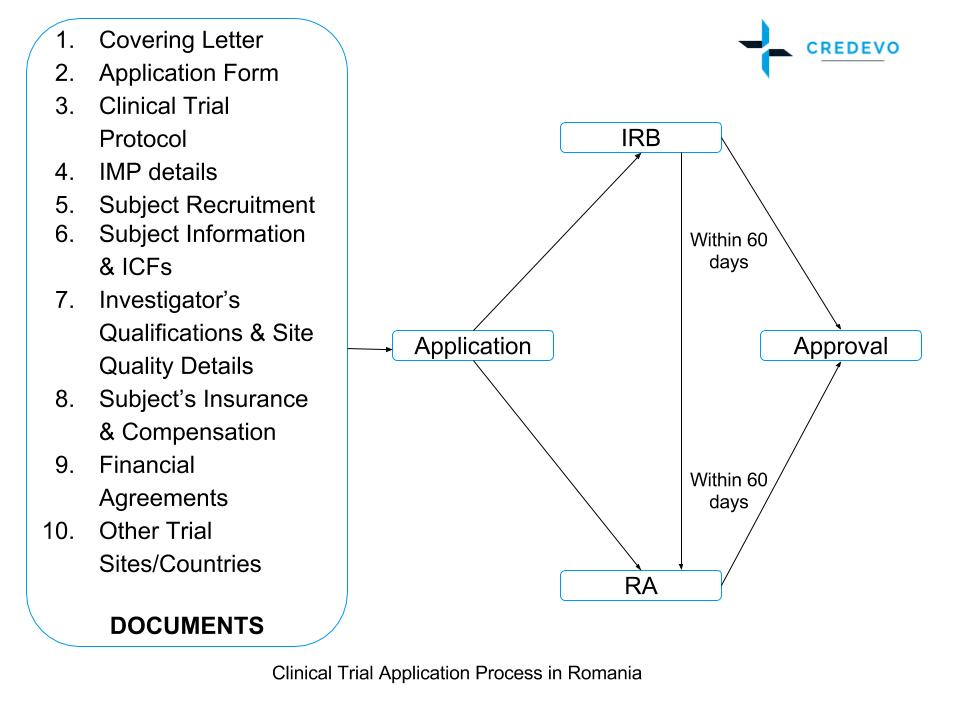
Clinical trial approval timelines
- The applicant will receive information about the validity of its request within 10 days of submission from RA.
- In case of appropriate documentation is submitted, RA will issue an authorization within a maximum of 60 days. The examination of an application submitted to the regulatory authority in the proper format will not take more than 60 days.
- In case of refusal, the applicant may request that the agency revise this within 30 days.
- The ethics committee’s opinion will also be issued within 60 days of a request.
The fee to conduct clinical trials in Romania
According to the authorization procedure for conducting clinical studies by the National Agency for Medicines and Medical Devices Scientific Council’s Decision 6/2014, the issuance of authorization for performing clinical studies is subject to the following fee structure:
- €1,250 for new substances,
- €1,000 for clinical investigation of medicines not authorized in Romania but authorized in other countries or authorized for marketing, when the study is conducted concerning aspects which fall outside the medicine’s summary of characteristics,
- €410 for studies performed on products authorized in Romania, used in conformity with their summary of characteristics, and
- €600 for bioequivalence studies.
Need Support for conducting your clinical trials in Romania or Have questions?
We’d love to help you conduct clinical trials in Romania.
Provide your details below.
Click on the useful links below
- How To Save Upto 80% Of Cost And Time In Initiating Clinical Trials
- Finding Clinical Trials to Work Upon as Clinical Investigator
References
- http://synevo-centrallabs.eu/media/docs/resources/JCS_March_2014_Clinical_Trials_in_Romania.pdf
- http://www.jforcs.com/wp-content/uploads/2015/05/09.-Romanias-Regulatory-Framework….pdf
- http://www.eurecnet.org/information/romania.html
- http://tangentdata.com/clinical_trials_in_romania
- http://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60372-3/fulltext
- http://www.efgcp.eu/Downloads/EFGCPReportFiles/Romania%20definitive.pdf
- https://www.lexology.com/library/detail.aspx?g=b85fa44a-4fd8-480e-9b5c-ddca500815ea
Do you have any comments? please do not hesitate to put them below here.
One thought on “Why Your Clinical Trials Should Include Sites in Romania?”
Comments are closed.