New Zealand – Clinical Trial Regulatory Process

New Zealand could be an interesting choice of region for national and international organizations seeking to pursue clinical trials and looking for the most preferred destination in the Asia-Pacific Region.

New Zealand, a country named the third most beautiful country in the world with stunning and diverse natural beauty, jagged mountains, is full of islands with one of Earth’s most peculiar bioregions, inhabited by flightless birds seen nowhere else such as a nocturnal, burrowing parrot and kiwi. Now, New Zealand is also known as the preferred country for conducting Clinical Trials.
Why Clinical Trials in New Zealand?
New Zealand has a great track record of early phase and proof of concept trials validated by independent, clean, and accurate clinical data.
Overwhelming factors:
- High-quality research facilities
- Efficient ethics and regulatory framework
- Can accommodate seasonal differences
- The diverse participant recruitment pool
Regulatory Authority in New Zealand
- The Director-General of Health requires approval before commencing a clinical trial using a new medicine in New Zealand.
- Health Research Council (HRC) – The Health Research Council (HRC) of New Zealand makes a recommendation to the Director-General on the clinical trial application.
- Medsafe – The application and approval process for clinical trials is administered by Medsafe.
- Health and Disability Ethics Committees – The Health and Disability Ethics Committees administer the ethics approval system, which applies to all clinical trials conducted in New Zealand.
Curious to understand the clinical trial approval process in Australia? Click here for insights.
Health Research Council of New Zealand (HRC)
- The Health Research Council of New Zealand (HRC) recommends the approval of clinical trials to the Director – General Health
- The HRC maintains two standing committees to consider clinical trial applications and make recommendations to the Director-General.
- Standing Committee on Therapeutic Trials (SCOTT) considers applications for new pharmaceutical-type medicines, and the Gene Technology Advisory Committee (GTAC).
What is Medsafe?
Medicines and Medical Devices Regulatory Authority for New Zealand is Medsafe.
It administers the regulatory application for clinical trials under Section 30 of the Medicines Act 1981, involving the use of new medicines, unregistered medicines, scientific assessment of gene technology, and medical devices. Medsafe issues approvals under a delegation from the Director-General of Health.
Medsafe receives and processes applications, liaises with the relevant Health Research Council committee (Standing Committee On Therapeutic Trials (SCOTT) or Gene Technology Advisory Committee (GTAC) and the applicant, and issues approval letters.
It also administers a notification scheme for clinical trial sites that have patients in residence and maintains a list of sites for which it has received notification of compliance with Good Clinical Practice requirements.
Health and Disability Ethics Committee (HDEC)
New Zealand has two types of approved Ethics Committee (EC) that conduct ethical reviews of clinical trials
- Most clinical trial approvals use HDECs, and
- Institutional Ethics Committees (IEC) handle late-phase research involving humans originating from that specific institution.
The New Zealand Health and Disability Ethics Committee (HDEC) administers the ethics approval system, which applies to interventional clinical trials regardless of whether they are trials that require approval under Section 30 of the Medicines Act.
Clinical Trial Sites in New Zealand
The application must include information about the site(s) at which the trial is to be conducted, and the facilities available at those sites. This information is also taken into consideration in deciding to approve the trial.
The person who manages the site (where study participants stay overnight) completes a Clinical Trial Site Notification form and should notify that the site has adequate emergency procedures in place.
On receipt of a completed notification, Medsafe will add the site to a list of Notified Clinical Trial Sites on the Medsafe website (http://www.medsafe.govt.nz/regulatory/CSSites.htm).
Clinical Trial Registry in New Zealand
The Australian New Zealand Clinical Trials Registry (ANZCTR) is an online public registry of clinical trials, held at the NHMRC Clinical Trials Centre, University of Sydney. It is a Primary Registry in the World Health Organization (WHO) Registry Network, which means it fulfills certain criteria for content, quality, validity, accessibility, unique identification, technical capacity, and administration.
The ANZCTR accepts both interventional and observational studies for registration from all countries and the full spectrum of therapeutic areas including trials of pharmaceuticals, surgical procedures, preventive measures, lifestyle, devices, treatment and rehabilitation strategies, and complementary therapies.
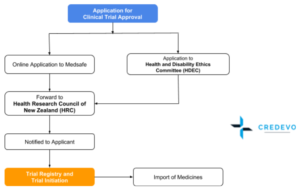
Clinical Trial Approval Procedure in NZ
- To obtain approval for clinical trials, applicants must use the online system. The system does not accept paper-based or emailed applications. You can submit an online application for clinical trial approval using the NZ Online platform at https://nz.ethicsform.org/signin.aspx.
- The applicant must first create an account to access SCOTT / GTAC and HDEC application forms.
- The person responsible for the clinical trial in New Zealand should make an application for approval. The applicant must be a person in New Zealand who takes legal responsibility for the conduct of the trial in New Zealand.
- On receipt of the online application, Medsafe will issue an acknowledgment letter and an invoice to the applicant within 7 days and Medsafe will forward the application to the Health Research Council of New Zealand (HRC). the applicant can communicate with Medsafe at [email protected]
- You can make an application for Ethics Committee approval at any time before, during, or after considering the application for clinical trial approval.
- Within 45 calendar days of receiving the application, the applicant will be notified about the outcome of the Director-General’s consideration of the HRC’s recommendation and will liaise with the applicant regarding any proposed conditions of approval or requests for further information.
- if the Director-General decides to approve the trial, a letter will be issued by Medsafe. If the decision is to decline an application, we will provide the reasons for this decision to the applicant. The applicant then has 28 days in which to appeal with the Medicines Review Committee.
Type of Approval
The process of approval for Clinical Trial is Parallel. The applicant can apply Medsafe along with HDEC
Time of Approval
The timeline of approvals is approximately 45 Days for the Director-General’s decision.
Import
Medicines and Treatments
- Before importing drugs into New Zealand, the trial requires approval from the Ministry of Health (Medsafe). You can use the Medsafe Standing Committee On Therapeutic Trials (SCOTT) Approval Letter as evidence of import approval if needed.
- There is no requirement for an import license. Additionally, investigational products shipped from the U.S. to New Zealand do not require export approval.
Materials used in Clinical trials
- All imports into New Zealand are subject to the Ministry of Agriculture & Forestry (MAF) and New Zealand Customs Import regulations.
- A commercial invoice is required to accompany all goods.
Clinical Trial Statistics in New Zealand
A total of 2,398 clinical trials have been registered in New Zealand with 298 ongoing trials
The major therapeutic areas in which most studies were registered are
- Gastrointestinal & Digestive system disease
- Oncology
- Infectious disease
- Respiratory disease followed by
- Heart diseases
Do you need support to conduct your clinical trials in New Zealand?
Engage with Credevo to discuss your clinical research needs that can be fulfilled in New Zealand. Learn more from our experts, and feel free to direct your questions to us.