Russia – Clinical Trial Regulatory Process

Russia has become an increasingly attractive venue for conducting clinical trials for international pharmaceutical companies. Russian clinics and principal investigators have proven to be highly capable of conducting clinical research at a level of quality not less than that of investigators in Western Europe and North America.

Clinical trials in Russia
Sponsors are considering Russia as an attractive venue for clinical trials due to the higher enrollment, patient retention rates, and lower overall cost. A recent report shows that sponsors are satisfied with the results of their clinical trials run in Russia however there remains unfamiliarity with the somewhat unpredictable regulatory environment.
Currently, all major pharmaceutical companies are preferring to conduct clinical trials in Russia and approve their products worldwide based on clinical data received from Russian investigational sites.
Are you looking for regulatory support in various stages of drug development? write to us at [email protected] or provide your query in the form below or or explore Credevo clinical development services
Top advantages of clinical trials in Russia
There are a multitude of factors that influence companies decisions to open clinical operations in Russia for all types of clinical trials: some of them are
Russia Clinical Trial Advantages
- Data acceptance
- Patient pool
- Relevant investigators’ expertise, and
- Cost-efficiency
Data acceptance
With adherence to global standards and regulations observed in clinical studies, The FDA and EMA accept data from such clinical trials in Russia.
Patient pool
The Russian hierarchical health care system contains a large number of health care facilities across the country which provides substantial access to various patient populations and enables rapid recruitment of study participants. Genetic diversity and high urban proportion
Investigators expertise
Access to educated, experienced, and compliant investigators who are motivated to participate in clinical trials to advance new drug development. There are GCP-trained and certified Investigative Sites generating high-quality data.
Lower Costs
Russia offers lower costs for conducting clinical trials as compared to the US and Western Europe. Some of the experts have claimed that compared to the US, Russian sites offer approximately 30-60% savings to sponsors depending on the complexity of the study, study-specific procedures, patient population, and etc.
Major Therapeutic areas
There are many unmet medical needs in the region, particularly in the treatment of non-communicable diseases and lifestyle disorders, such as
- Cardiovascular diseases,
- Diabetes and
- Cancer followed by other therapeutic areas
Regulatory bodies
Main regulatory bodies in Russia for clinical trial processes are as follows
- Ministry of Health Care and Social Development
- Federal Service for Supervision in Health Care and Social Sphere
- Scientific Center of Expertise of Medicines and Medical Devices
- National Ethics Council
- Medical Institutions
Ministry of Health Care and Social Development
Takes care of
- Accreditation of medical Institutions,
- Approval of clinical trials,
- Information services related to clinical trials,
- Relations with the medical community
Federal Service for Supervision in Health Care and Social Sphere
Takes care of
- Supervision of clinical trials,
- Inspections in clinics,
- Pharmacovigilance
Scientific Center of Expertise of Medicines and Medical Devices
Undertakes review of clinical study documents
National Ethics Council
Responsible for ethics supervision of clinical trials
Medical institutions
Responsible for GCP compliance, Clinical trials reports, Local ethics committees, and SAE reporting
Requirements & process
Any company that plans to conduct clinical trials in Russia should have robust support from a locally based regulatory team that has a deep understanding of the requirements and is aware of any regulatory changes.
Documents for authorization of Clinical Trials
- Application letter
- Proof of payment of state duty
- Report on preclinical trials of the medicinal product and report on earlier clinical trials of the investigational drug (if any).
- Clinical trial protocol
- Investigator’s Brochure
- Informed consent form and information sheets of the patients
- Data about the investigators’ work experience in the relevant specializations and their clinical trial experience
- Information about health care institution, where the clinical trial is to be conducted
- Information about proposed timelines for the clinical trial
- Copies of policies for compulsory life and medical insurance of patients to be enrolled in the clinical trials
- Information about the composition of the investigational product
- A document by the manufacturer providing data about the characteristics (parameters) of the investigational product manufactured for the clinical trial.
Requirements for Institutions doing CTs
- Accreditation by MoH License for medical activity
- Intensive care unit if phase I studies are planned
- Presence of copies of legislative documents regarding CTs
- Compliance with GCP
- Protection of confidential information
Requirements for investigators
- 5-years experience in clinical trials as a PI
- Specialization is relevant to the field of the trial
- Submitted CV (template is available) should contain the following
- Information about CT experience:
- Protocol ID and title in Russian, phase, timelines
- Number of regulatory approval of the CT
- PI’s name if the applicant was a co-investigator
- Document proving participation in the study as co-investigator
- Registry of investigators will be available in the Internet
Electronic application requirements
- Detailed application form on the website of MoH (http://grls.rosminzdrav.ru/registrate.aspx)
- All submitted documents should be attached
- Electronic application duplicates the paper one
- Currently, the electronic application is requested before the final stage of approval
Ethics Committee
- There are two levels of ECs in Russia, federal level (central level) and local/institutional.
- The sponsor is responsible for submitting the request for the expert ethical opinion to the ‘Council on Ethics’, a subordinated organization within the Ministry of Health and Social Development, as part of the application for the clinical trial permit.
- The principal investigator is responsible for submitting the request to the local (institutional affiliated) research ethics committee. [6]
Process of approval
- The process starts with compiling all the required documents and submitting them to (MOH) Ministry of Health Care and Social Development.
- The Department of State Regulation of Drug Circulation checks the submitted documents for completeness and issues a written acceptance notification to the applicant within 5 working days.
- The study application after a review of the completeness of the submission is forwarded to NEC (National Ethics Council) & (SCEMP) Scientific Center of Expertise of Medicines and Medical Devices or return if the application is lacking details or incomplete
- Both subordinated organizations review the documents
- Ethics Council (NEC) gives its opinion on the approval/rejection, and
- Expert Organization (SCEMP) provides its expert inputs sourced from experts in CMC, toxicology, pharmacology, clinicians, etc.
- At the federal level, the ethics & expert review is to be completed within 35 days (which is hardly ever met actually, and even timelines of 75-120 days have been reported).
- If the application is approved by the concerned bodies, it is forwarded to the Ministry of Health Care and Social Development.
- An approved study is informed to the applicant. [5][6]
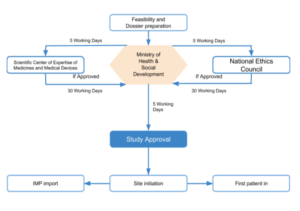
Approval timeline
The approximate approval timeline is 2 -4 months depending on the case.
Do you need any support for your clinical trial in Russia?
Provide your requirement details below to connect with us and explore our clinical development, monitoring, and regulatory support in Russia
References
- http://www.biorasi.com/assests/white-papers/Biorasi%20-%20Navigating%20through%20the%20Clinical%20Trial%20Authorization%20Process%20in%20Russia-v0-1.pdf
- http://www.jforcs.com/wp-content/uploads/2017/02/Clinical-trials.pdf
- http://www.fisherclinicalservices.com/en/learning-center/insights-blog-overview/7-best-practices-in bringing-a-clinical-trial-to-russia.html
- https://premier-research.com/wp-content/uploads/2014/12/Experience_Russia_201206.pdf
- https://clinicaltrialsarena.com/news/clinical-trials-arena/exploring-considerations-to-help-carry-out-clinical-trials-in-russia-5709878-2/
- https://www.nihcollaboratory.org/sites/CbyC/Pages/SnapshotDrug.aspx