Sri Lanka – Clinical Trial Regulatory Process

Sri Lanka was one of the first countries to embrace the concept of clinical trial registration. The SLCTR was recognized as a Primary Registry of the Registry Network of the WHO‐ICTRP in March 2008, being the fourth Primary Registry to join the Network.

Why Sri Lanka?
Sri Lanka is an emerging market for clinical trial business with some contract research organizations already operational in the country.
The availability of large numbers of patients, well-trained clinicians, a good system of ethics review, and an efficient regulatory process implemented through the Drug Regulatory Authority of the Ministry of Health makes it possible to run clinical trials in Sri Lanka according to ICH-GCP guidelines. [1]
Clinical Trial Regulatory in Sri Lanka
The Sub Committee on Clinical Trial (SCCT) was established under the Cosmetics Devices Drug Regulatory Authority (CDDRA) of Sri Lanka. It provides regulatory approval as well as issue relevant license and certificates required for the conduct of clinical trials, ensures implementation of Good Clinical Practice (GCP) standard in the conduct of Clinical Trials while monitoring the safety & wellbeing of clinical trial subjects. [2]
It has also released guidelines for the conduct of clinical trials in Sri Lanka [4]. Check out this guideline here for more details.
Ethics Committee
The Ethics Review Committees (ERC) ensures the study proposals comply with internationally accepted guidelines standards and the research subjects are adequately protected.
The following eight institutional ERCs are recognized by the SCOCT and ethical approval must be obtained from at least one of them.
- Ethics Review Committee, Faculty of Medicine, University of Colombo
- Ethics Review Committee, Faculty of Medical Science, University of Sri Jayewardenepura
- Ethics Review Committee, Faculty of Medicine, University of Kelaniya
- Ethics Review Committee, Faculty of Medicine, University of Ruhuna
- Ethics Review Committee, Faculty of Medicine, University of Jaffna
- Ethics Review Committee, Faculty of Medicine, University of Peradeniya
- Ethics Review Committee, Medical Research Institute, Colombo
- Ethics Review Committee, Sri Lanka Medical Association [3]
Srilankan Registry
The Sri Lanka Clinical Trials Registry (SLCTR) is a central repository of key information about Registry for clinical trials involving human subjects, conducted in Sri Lanka or overseas. It is a not-for-profit Registry, with free and open access to researchers, clinicians, and the general public.
Any investigator, from Sri Lanka or overseas, can register a clinical trial with the SLCTR.
Trial registration can be done by completing the Application Form on-line. All data fields must be completed by the investigator. It is assumed that all trials would have obtained approval from a recognized ethics review committee before enrolment of subjects.
For more details about the registry, you can visit the link SriLankan Registry
The fee varies based on the type of trials
- Investigator funded trials Rs. 5,000/-
- Grant-funded trials (Government or Research Grants) Rs. 10,000/-
- Pharmaceutical Industry-Sponsored Trials Rs. 25,000/-
- International investigator funded trials USD 100
- International Pharmaceutical Industry-Sponsored trials USD 250
For more details and inquiry you can contact at [email protected] or by phone at (+94 112690212) [3]
Requirements for Clinical Trial Approval
- Approval from the Sub-Committee on Clinical Trials (SCOCT), Cosmetics Devices & Drugs Regulatory Authority (CDDRA), Ministry of Health.
- Clearance from Ethics Review Committees (ERC)
- Approval or a No-objections certificate from the head(s) of the institution(s) of the trial site(s) (e.g. Director of a hospital)
- Registration of study in the Sri Lanka Clinical Trials Registry
- Clinical Trial Protocol (Version & date)
- Investigator brochure (Edition & date)
- Patient information documents and patient informed consent form (ICF) in English along with Sinhalese and Tamil translation and back translations
- GMP certificate & Certificate of analysis for manufactures
- Listing of overseas trial centers and regulatory approvals if relevant Principal investigators’/ coordinating PI’s curriculum vitae
- Product liability letter or insurance certificate
Type of Approval
Submissions can be made Parallelly to both the SCOCT and to the relevant ERC. However, SCOCT approval to conduct a clinical trial will be granted only after clearance from ethics committees. [3]
Regulatory Process in Sri Lanka
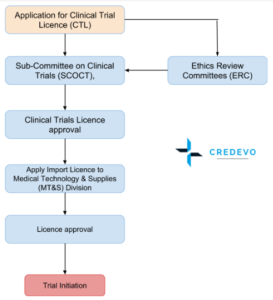
- To conduct clinical trials (Clinical Trial Licence – CTL), an application must be made (in triplicate) by the sponsor/Investigator or coordinating investigator in case of multi-center studies to the Sub-Committee on Clinical Trials (SCOCT), CDDRA
- The regulatory approval should be sought for Clinical trials when,
- Using drug which is not registered in Sri Lanka (NCEs)
- Using a registered drug for a new indication.
- For the multisite clinical trials in Sri Lanka, only a single application is sufficient to be submitted to the SCOCT.
- The SCOCT will not issue an approval unless clearance from an Ethics Review Committee accredited by SCOCT.
- On review of all submitted documents, an approval to conduct a clinical trial is issued by the SCOCT which may be subject to terms and conditions as the SCOCT may impose.
- An approval to conduct a clinical trial will be issued in the name of the applicant and the clinical trial should be conducted only at the site(s) specified in the approval.
- Approval granted by SCOT will have a validity of 5 years which can be extended when the user applies before 60 days from the expiry of approval.
- Upon receiving approval for the clinical trial from SCOCT, the holder of the approval may apply to the Medical Technology & Supplies (MT&S) Division, CDDRA, Ministry of Health, for an import license and other permits that may be necessary for the import of investigational medicinal products and other drugs and material required for the study. [3][4]
Approval process flow
Requirements to be fulfilled after approval
- Registry: It is mandatory to register all clinical trials in a primary registry of the WHO clinical trial registry network before enrolling patients in the study.
- Sri Lanka Clinical Trials Registry (SLCTR) of the Sri Lanka Medical Association (SLMA) is a primary registry recognized by the WHO.
- Reporting: To submit the progress report every six months and at termination or completion of the study
- To report any changes or deviation of the approved protocols
- To report all serious adverse events and new information that may affect adversely the safety of the subjects or the conduct of the trial. [4]
Import & Export Licence
- Holder of the approval may apply to the Medical Technology & Supplies (MT&S) Division, CDDRA, Ministry of Health, for an import license and other permits
- Products that are not registered with the Cosmetics Devices and Drug Regulatory Authority (CDDRA) and placebos
that is intended to be imported for the purpose of conducting a Clinical Trial. - After regulatory approval is obtained, the applicant can apply for a license to import each consignment of the investigational drug. [4]
Do you need our regulatory services for your clinical trial in Sri Lanka?
Think about Sri Lanka as a huge support for your south Asia strategy of clinical development. It could be helpful in several ways.