Clinical Trial Regulatory Process – Mexico

Mexico is rapidly becoming a major player in the pharmaceutical and medical device industries. A recent report states that It’s the number one exporter of medical devices in Latin America and 8th in the world and also a leading exporter of pharmaceutical products in Latin America.

Having a diverse demographic profile gives the enormous potential for the recruitment of patients on a large scale. Researchers have experience in all therapeutic areas and population groups with experience in implementing a diverse epidemiological profile.
About Mexico
Located between the United States and Central America, Mexico’s geography and Spanish language put it in an ideal position to act as a gateway to Latin American pharmaceutical markets.
With an estimated size of $10 billion in 2016, the country’s pharmaceutical market ranks twelfth globally with a stable economy although it is classified as upper-middle-income, it also offers lower costs compared with other countries.
Clinical Trial Overview
- Clinical Trial Application language is Spanish
- The type of approval is Sequential
- An in-country Representative or Sponsor is required
What is the Clinical trial Regulatory in MEXICO?
Any research on human beings which includes testing new medicinal products and new uses, as well as dosages and administration routes for already approved medicinal products are approved by the Federal Commission for Protection Against Sanitary Risk (COFEPRIS – Comisión Federal para la Protección contra Riesgos Sanitarios).
The Federal Commission for the Protection against Sanitary Risk (COFEPRIS) is classified as a Level 4 national regulatory authority by the Pan American Health Organization/World Health Organization (PAHO/WHO) and has signed bilateral agreements with other regulatory agencies that recognize registrations made by COFEPRIS.
Clinical trials for innovator biological products must take place in Mexico for the product to be manufactured in Mexico. For products manufactured abroad, the Ministry of Health can request a clinical trial in Mexico when the Sub-Committee on Evaluation of Biotechnological Products of COFEPRIS considers that this is necessary.
For any regulatory inquiries, the applicant can visit Integral Services Center or [email protected], [email protected]
Ethics Committee
- Mexico has a decentralized registration process for Research Ethics Committees (REC) operating through the National Bioethics Commission
- REC – Research Ethics Committee and RC – Research Committees approval is required for each trial site where a study is being conducted, and when applicable, Biosafety Committees (BC) approval is required as well
- Research Committees (RCs) and Biosafety Committees (BC) within each health institution must register with the Federal Commission for the Protection Against Sanitary Risks (COFEPRIS) where the study is being conducted
- REC must approve the informed consent document and evaluates the technical quality and scientific merit of the proposed research.
- The BC is responsible for determining and regulating the use of ionizing radiation or genetic engineering techniques within the health institution
- The REC and COFEPRIS reviews may not be conducted in parallel.
Site Selection
- The sponsor is responsible for selecting each research center and ensure that COFEPRIS has authorized the site for operation
- The sponsor or his/her contract research organization (CRO) should comply with the Guideline for Good Clinical Practice.
What are the requirements for approval?
All documentation related to submitting applications for research protocol authorization is required to be in Spanish, no documentation (e.g., protocol and researcher’s manual) should be submitted in English.
- Approval from an independent ethics committee registered with the Ministry of Health;
- Approval from the medical institution or institutions where the clinical trials will be conducted;
- COFEPRIS approval for institutions to conduct clinical trials;
- Clinical trial protocol (including a schedule and the approximate amount of medicinal products to be imported);
- Written informed consent templates;
- Pre-clinical and clinical data that justify conducting the research;
- Description of available resources to conduct the research and to address emergencies (including a statement of sponsorship) and
- A written letter by the qualified investigator acknowledging his or her responsibilities, and data from the qualified investigator and his or her staff.
What is the type of Approval?
The type of approval is Sequential, where the Sponsor or applicant should first obtain approval from Ethics before getting approval from COFEPRIS.
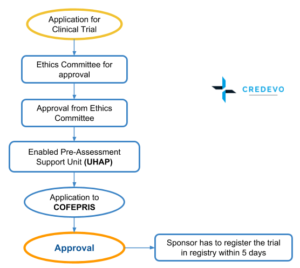
Process for Approval
- Step 1: The process starts with an evaluation of the proposed trial at the host institution by the respective Ethics committee, Research committee, and Biosafety Committee. This step may take between three and six months.
- Step 2: The second step may have two different Processes. In the most established process or pathway, COFEPRIS makes an analysis and decision, which may take up to 90 days.
- Rather than submitting the application directly, the applicant can first choose to obtain a pre-assessment evaluation of the application through an Enabled Pre-Assessment Support Unit (Unidad Habilitada de Apoyo al Predictamen (UHAP) fee for which is 60,000 Mexican Pesos
- The advantage of this additional evaluation is that once a UHAP issues a favorable letter of pre-authorization, which may help in guidance and possibility for rejection for the applicant in COFEPRIS and may include this response with submission to COFEPRIS through the CIS to be reviewed by the EC
- Receipt of a favorable pre-evaluation assessment also requires the EC to issue a response within a maximum of 30 business days as long as the applicant notifies the EC that his/her application was submitted with the pre-evaluation assessment.
- If the applicant selects a scientific committee within an institution that has a UHAP and the cost is 40,000 Mexican Pesos. The cost for each amendment is 3,500 Mexican Pesos, and corrections to the pre-assessment document are free.
- This newer pathway which uses Enabled Support Units for Preauthorization (UHAPs) will review and preauthorize trials in 45 days, followed by a COFEPRIS analysis that must offer a decision in 30 days.
- Once COFEPRIS issues an approval, the applicant has a maximum of five (5) working days to enter this information into the National Registry of Clinical Trials (Registro Nacional de Ensayos Clínicos (RNEC) and importation of new drugs needs to be authorized, which may take an additional 15 days.
UHAP: Pre-Assessment Support Unit (Unidad Habilitada de Apoyo al Predictamen,
COFEPRIS: Comisión Federal para la Protección contra Riesgos Sanitarios – Federal Commission for Protection Against Sanitary Risk
What is the Timeline for approval?
- COFEPRIS must approve a request for research protocol authorization within 30 working days from the day following an application’s filing.
- According to COFEPRIS, currently takes on average 10 months. The process starts with an evaluation at the host institution by the respective ethics committee, research committee, and biosafety committee where this step may take between three and six months.
Fee structure
- Research Ethics Committees (RECs) do not charge sponsors/investigators for review.
- The health institution must finance REC operating expenses, without this causing any conflict of interest in the committee’s functions.
- For fee and other required details, the applicant can visit Integral Services Center
Bioequivalence and Bio-analysis studies
Bioequivalence
- The pharmaceutical market in Mexico is divided into patented medicines 51 percent, generics with 35 percent, and OTC products with the remaining 14 percent of the market by value. According to the Federal Commission for the Protection Against Sanitary Risks (COFEPRIS), the country’s regulatory authority for the sector, generics represent more than 80 percent of the market in terms of volume.
- Mexico regulations for registration of generic products require proof of therapeutic bioequivalence in order to allow health authorities to declare interchangeability between the non-innovator product (the generic product) and the reference product.
- A company can submit a dossier for approval of a biocomparable to COFEPRIS eight years prior to the expiration of the patent on the originator biological, however, approval for the biocomparable will only be granted at the end of the term of the patent.
- Mexico does not accept foreign comparators and accept bioequivalence studies involving only their local comparator products
- Companies have to provide information concerning dissolution profiles or bioavailability studies regarding the reference product. COFEPRIS periodically issues a list of reference medicinal products.
Bio-analysis
- The Federal Commission for the Protection against Sanitary Risk (COFEPRIS) is in charge of regulating BA laboratories
- According to Mexican regulations, all steps for a BE study should be conducted inside the country.
- A ‘restraint of trade’ is likely to rise, which means that BA laboratories outside the country could be authorized to perform analysis by Mexican authorities.
Dossier requirements for submission to Regulatory bodies
- Dossiers to be submitted in local language
- CPP / WHO GMP / Manufacturing license
- Free Sale Certificate
- Letter of Authorization / Power of Attorney
- Legalization of administrative documents from the embassy
- API Technical package (Brazil, Mexico)
- Specification and methods
- COA of API and Excipients from vendors
- Manufacturing procedure and controls
- Executed Batch manufacturing records / Batch Numbering system.
- Stability data on three batches of Stability conditions as per zone definitions.
What about Good Clinical Practice – GCP?
GCP in Mexico is well regulated. Although the regulation is robust, there is scope for improvement. Currently, efforts are in place to reach international standards.
What are the Insurance requirements for Clinical Trials?
The clinical trial budget should include compensation to which the subject of the investigation will be legally entitled in case of damages directly related to the clinical trial. Where appropriate, this financial fund can be covered under study insurance.
Import and Export of Biological Specimens
- Applicant should submit an import/export permit in print or electronically. For details visit Import and Export
- Institutions that dispense or import products of human beings including tissues, cells, blood, and its components must comply with specific COFEPRIS documentation submission requirements to apply for an import permit.
Clinical Trial Numbers Overview
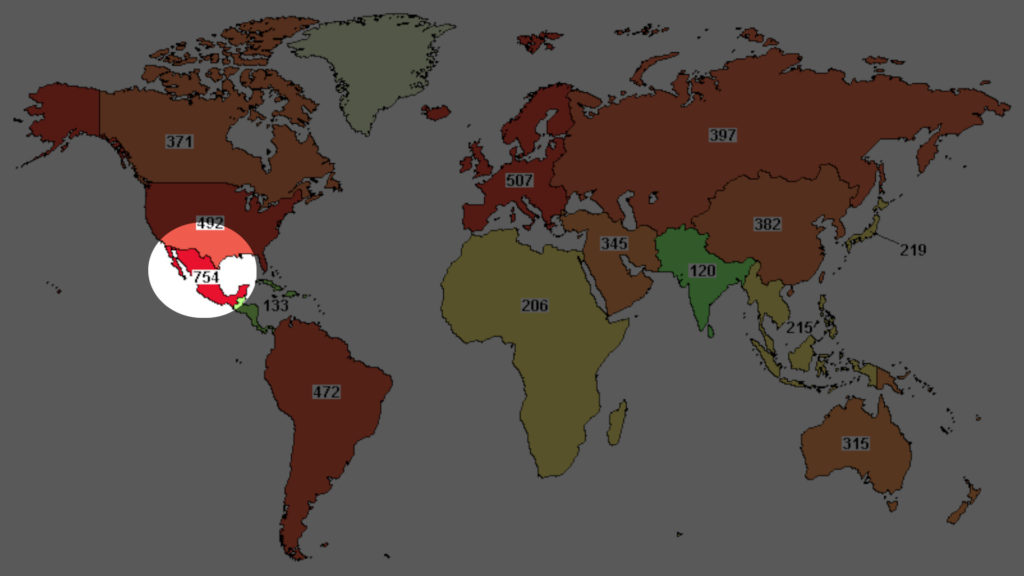
A total of 3282 studies were registered till date with 754 Ongoing studies.
Major therapeutic area of studies are
- Immune disorders
- Metabolic disorders
- Respiratory diseases &
- Communicable diseases
Need more info on Mexico?
Discuss with Credevo about your needs for clinical research that could be fulfilled in Mexico. Know more from our experts. Shoot your questions to us.
Reference
- https://www.medpace.com/spotlight-on-mexico/
- https://www.clinicalleader.com/doc/can-mexico-become-a-regional-powerhouse-for-clinical-trials-0001
- https://www.lexology.com/library/detail.aspx?g=fbe74b04-fe97-4340-8e18-bbc0dbc1b36d
- https://clinregs.niaid.nih.gov/country/mexico#vulnerable_populations
- http://new.paho.org/hq/dmdocuments/2008/WG-BE%20document%20eng%20221008.pdf
- https://www.future-science.com/doi/full/10.4155/bio.11.67
- https://clinicaltrials.gov/ct2/results?recrs=ad&cntry=MX&map_cntry=MX