Why and How To Start A Clinical Trial In Vietnam?

Vietnam, known for its tourism destination, is also a destination for clinical trials and research. With a sizeable population of 85 million, Vietnam offers a vast pool of participants, healthcare professionals, and strong doctor-patient relationships, which result in a greater retention rate.

Clinical Trials in Vietnam
In the last few years, Vietnam has been a preferred site for many clinical trials planned in South-East Asia. Along with the Philippines and Thailand, Vietnam is one of the ‘hot’ and upcoming countries for conducting clinical trials in ASEAN.
A total of 755 clinical trials were registered in Vietnam, 204 of which are ongoing or active trials. Vietnam has gained importance recently from clinical research companies, and the ratio of ongoing to total trials indicates a big positive shift in attention. (clinicaltrials.gov).
[last updated in Apr 2023]
Top therapeutic areas to conduct clinical research in Vietnam
Most of the trials in Vietnam are focused on certain specific therapeutic areas. The therapeutic segments to consider for clinical trials in Vietnam are as follows.
- Communicable and infectious diseases
- Respiratory and lung disease
- Gastrointestinal disease
- Immune disorders
- Metabolic and liver diseases
- Neurology
How to start your clinical trial in Vietnam?
For those, who would like to conduct a clinical trial in Vietnam for the first time, it is crucial to understand the regulatory, ethics, and local concerns around starting a clinical trial in Vietnam.
First and foremost, the regulatory area.
Clinical trial regulatory
The regulatory authority responsible for clinical trial approvals, oversight, and inspections in Vietnam is Vietnam’s Ministry of Health (MOH). Further, based on the specific area the two regulatory bodies are
- The national-level ethics committee (EC), the Ethical Evaluation Committee in Biomedical Research (EECBR), is responsible for protocol approval and study documentation.
- The Administration of Science, Technology, and Training (ASTT) reviews clinical study documents, organizes the Ethical Evaluation Committee in Biomedical Research (EECBR) meeting, and conducts its review of the Investigator’s Brochure (IB) portion of the dossier.
- ASTT is also responsible to register contract research organizations (CROs) that support clinical studies and provide other research services.
Clinical trial registration is not required.
The scope of the MOH’s assessment
The scope of the MOH’s assessment includes all clinical trials (Phase I-IV) for the following
- Newly developed biologics or biologics with a new combination of marketed ingredients.
- Drugs that are newly manufactured and used for the first time.
- Oriental medicines or traditional drugs, made from medicinal materials containing ingredients to use in humans for the first time.
- Drugs, biologics, and vaccines are marketed for less than five years in the country of origin.
- Drugs, biologics, vaccines, medical devices, combination products, and oriental medicines have clinical trial data that have not met the MOH’s or internationally recognized good clinical practice (GCP) requirements.
To begin the regulatory process, the sponsor must select the investigators and host institutions to conduct the clinical trial. This process is not very different from the clinical trial site selection process employed elsewhere.
Site Selection and Investigator
Vietnamese regulations require the sponsor to select investigator(s), principal investigator (PI), consultant experts, and host institutions. The sponsor shall consider the selection process based on the appropriateness and availability of the study site and facilities.
- Clinical trial sites are in northern and southern regions in and around Hanoi and Ho Chi Minh City, Vietnam.
- These sites, along with many other sites, provide a significant pool of patients for clinical trials.
- It might help to get local or expert support in reaching these sites.
Credevo provides exhaustive coverage of sites within Vietnam. More than 300 investigators within this small country are accessible through Credevo.
Interested to conduct clinical trials in Vietnam?
Provide brief details of your requirement in the below form and Credevo will help you get started.
The sponsor can initiate a clinical trial and EC approval process after choosing investigators/host institutions to conduct clinical trials.
Now, first, let’s understand a bit more about EC processes in Vietnam.
Ethics Committee
To conduct a clinical trial in Vietnam, the sponsor shall obtain approval from the institutional and national level ethics committee (EC).
- An EC registered as a Council of Ethics in Biomedical Research at the Grass-Root Level (CEBRGL) with the MOH’s ASTT provides institutional-level EC approval.
- The MOH’s Ethical Evaluation Committee in Biomedical Research (EECBR) conducts national-level EC approval.
- The EECBR’s activities conduct plenary meetings, subcommittee meetings, and periodic and ad-hoc investigations.
- CEBRGLs and the EECBR and are responsible for ensuring independent, timely, and competent reviews of all ethical aspects of the clinical trial protocol.
Regulatory approval process
Since the national EC as well as the RA approval process is performed together by MoH, it helps a lot to understand the RA process.
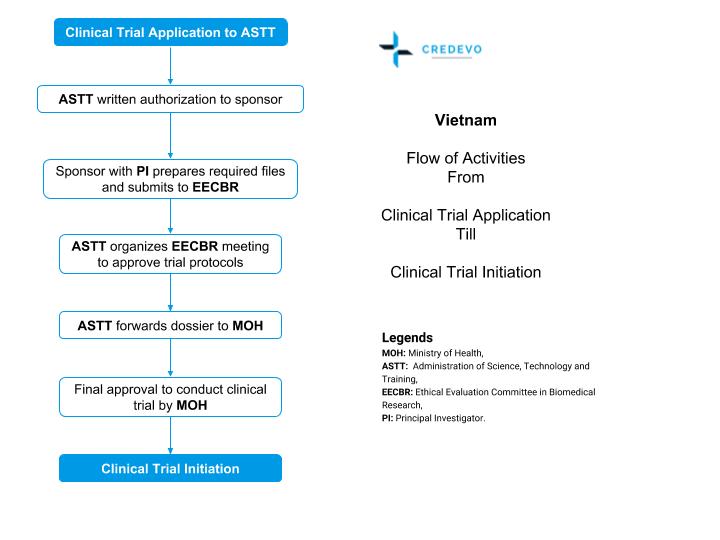
First, the sponsor is required to submit the following documents to ASTT
- Registration files that include the clinical trial application (CTA) form.
- Principal investigator (PI) details,
- Host institution proposal and
- Investigator’s Brochure (IB)
Upon receipt of the appropriate files,
- ASTT will respond to the sponsor in writing within 15 working days.
After clearance of registration files,
- The sponsor is responsible for coordinating with the principal investigator to prepare the required clinical trial files. The sponsor should also submit the IB to ASTT.
- The authority reviews the application received before the 20th of that month. The regulatory authority reviews the clinical trial applications submitted after the 20th of the following month.
Upon receiving all required documents,
- ASTT will assign a group to review the IB and will simultaneously organize the EECBR meeting to review and approve the protocol and other trial files.
After receiving the IB and the EECBR’s reviews,
- The ASTT will finalize the dossier and send written notice of the results to the sponsor, the principal investigator, and/or the host institution.
- ASTT completes the review once it receives the national-level EC approval.
- After approval, the files will be sent to the MOH minister who issues the final approval to the entire dossier.
The entire MOH review and approval process takes 60 working days approximately.
Type of application process
The ASTT conducts review in parallel with the EECBR review as this helps to minimize approval time.
Application language and exceptions
The official language is Vietnamese.
English or Vietnamese is acceptable for specific documents, these include:
- Sponsor-prepared clinical trial application form (English)
- IB (English and Vietnamese)
- The main study site-prepared clinical trial application form (English and Vietnamese)
- The study protocol (English and Vietnamese)
- Informed Consent (IC) form (Vietnamese and English)
- Drug manufacturing procedures (Vietnamese and English).
However, the study information sheet should be in Vietnamese.
Approval timeline
The entire MOH review and approval process takes approximately 60 working days from the date the sponsor and the principal investigator submits documents to the ASTT and the EECBR.
MOH minister issues final approval in about 10 working days.
Processing fee
- EECBR currently charges a fee of $1,000-$2,000 USD.
- EC is registered as a Council of Ethics in Biomedical Research at the Grass-Root Level (CEBRGL) and charges a fee of $500-$2,000 USD depending on the complexity of the trial.
- For locally-manufactured medical devices, a fee of 300,000 VND per dossier, and import permits are 200,000-3,000,000 VND.
Trial initiation
After necessary approvals from ASTT and national-level EC have been secured, it becomes possible for the clinical trial management team (from the sponsor or CRO) to initiate the clinical trial. However, at this stage, it’s necessary to have permission to import any medicinal drugs and other supplies.
Import License
The sponsor should obtain an import license for the shipment of an investigational product used in the trial from the MOH’s Drug Administration of Vietnam (DAV).
Letter of Agreement (LoA)
After securing EECBR approval, the sponsor needs to sign a Letter of Agreement (LoA) with the participating host institution(s) before the trial begins.
Safety reporting
After clinical trial initiation, Vietnamese regulations require investigators to submit periodic safety updates to the national ethics committee (EC) – EECBR.
The principal investigator must send progress reports of the trial to the sponsor and EECBR, every 3 months from the date of the trial’s initiation.
This report should contain the following
- Investigational product (IP) safety and side effects
- Serious Adverse Events (SAEs)/Serious Adverse Drug Reactions (SADRs)/Suspected
- Unexpected Serious Adverse Reactions (SUSARs).
Have any questions or need support?
Do you have any questions or need our support in conducting clinical trials in Vietnam? Please provide your requirements below to connect with us and explore our services.
To explore more South East Asian regions, follow the links below
- Clinical Trials in Indonesia
- Malaysia – Why and How to Start Your Clinical Trials?
- Philippines – Clinical Trial Regulatory Process
- Singapore Clinical Trial Regulatory Process
- Thailand’s Clinical Trial Regulatory Scenario – Simplified (Part – 1, Part – 2).
2 thoughts on “Why and How To Start A Clinical Trial In Vietnam?”
Comments are closed.