Choosing Asia-Pacific For Your Clinical Trials: Strategic Insights

The Asia-Pacific region emerges as a pivotal player in the landscape of clinical trials. They offer unique advantages for conducting impactful research due to diverse populations, varied healthcare infrastructures, and a rich pool of treatment-naive patients.

Over the past decade, there has been a notable shift in the global Phase I clinical trials scenario, with the Asia-Pacific (APAC) region becoming a focal point. Between 2013 and 2022,
APAC emerged as the fastest-growing region for early-stage clinical trials, experiencing a combined annual growth rate 12 times greater than the United States and four times higher than Europe. In 2022, APAC claimed a significant share, contributing to 58% of all global Phase I clinical trials.
As we delve deeper, we will unravel the factors that contribute to the global significance of this region in clinical trials, providing strategic insights for those navigating this dynamic field.
Why is Asia-Pacific looking promising to conduct clinical trials?
The Asia-Pacific region is looking promising to conduct clinical trials due to several factors. The large and diverse population and lower costs for conducting clinical trials make it a favorable research environment. Moreover, many countries in this region have well-established healthcare infrastructure and research capabilities, contributing to the global clinical trial landscape.
Let’s take a closer look at each factor.
Rapid market entry
- Since 2018, the Asia-Pacific (APAC) region has excelled in Phase I clinical trials with the shortest patient enrollment duration, thanks to its extensive patient base.
- The median enrollment time in APAC was twice as fast as in Europe and three times quicker than in the United States. APAC achieved a median recruitment rate of 22.47 subjects per site per month, making it three times faster than Europe and seven times faster than the United States.
- This implies that sponsors experience quicker trial initiation, leading to reduced overall trial duration and faster market availability for new drugs.
Access to a large pool of patients
- The Asia-Pacific (APAC) region, with its 4.3 billion residents, attracts clinical trials due to its large population.
- Many APAC nations have quality medical infrastructure and efficient application processes. The trial data is renowned for its quality, aiding approval in other markets.
Cost-effective
APAC stands out for its budget-friendly environment in clinical trials, with lower operating costs compared to other regions. This affordability may lower trial expenses and increase their effectiveness.
Availability of skilled talents
The Asia-Pacific region boasts skilled professionals well-versed in global regulations. It houses advanced clinical trials and disease centers with expertise.
Easily obtainable quality data
APAC countries uphold high data quality standards, particularly in Korea, where universities use advanced digital data capture methods surpassing typical clinical trial practices. Other regions are also adopting cutting-edge data-capture techniques.
Moving with the market
- APAC leads Phase I studies in therapeutic areas like oncology, infectious diseases, the central nervous system, cardiovascular disorders, and metabolic disorders.
- Between 2018 and 2022, APAC had the highest trial numbers compared to Europe and the US, with China contributing over 80% in these areas.
- The region also hosted most Phase I trials for biologic drug therapies, including immunomodulatory, immune-oncology, adoptive cell therapy, vaccines, and gene-based therapies, accounting for significant percentages in various categories.
The landscape
- Over the last decade, APAC has become a hub for both local and international drug development companies searching for new drug opportunities.
- The primary countries for Phase I clinical trials in Asia include China, Australia, South Korea, Japan, and India.
- These nations are gaining recognition for their exceptional research, collectively contributing to over 50% of global Phase I trials, with China leading at nearly 40%.
Are you aware that regulatory bodies in the Asia-Pacific region offer incentives for clinical trials? Countries such as South Korea, Japan, Australia, China, and India provide financial benefits and incentives for orphan drug developers. Click on the countries to learn more.
What are the challenges when choosing an Asian Pacific country to conduct a clinical trial?
Selecting an Asia-Pacific country for a clinical trial presents unique challenges.
- Firstly, cultural differences demand careful consideration, as diverse customs can impact participant engagement.
- Language barriers pose a hurdle, necessitating accurate translation of study materials for participant comprehension.
- Collaborating with local experts becomes crucial to navigating regional healthcare intricacies and ensuring successful trial implementation.
Cultural differences
Conducting a clinical trial in the Asia Pacific region requires sensitivity to diverse cultures. Varied customs, beliefs, and healthcare practices may impact participant recruitment.
Language barriers
Language diversity poses challenges in communication between researchers and participants. Ensuring accurate translation of consent is crucial to maintaining ethical standards and participant comprehension.
Collaboration with local experts
Successful trials involve collaboration with local healthcare professionals. Building a partnership ensures a better understanding of regional healthcare infrastructure, improving trial design and participant recruitment.
Time zone differences
Managing trials across different time zones demands meticulous planning. Coordination for data collection, virtual meetings, and real-time communication necessitates effective scheduling to overcome logistical challenges.
Do you need support in conducting your clinical trials in the Asia-Pacific region? Please provide your requirements below to connect with our expertise.
Which countries in the Asia Pacific region are the primary choices for conducting clinical trials?
- Several countries in the Asia Pacific region are primary choices for conducting clinical trials due to their well-established healthcare infrastructure and regulatory frameworks.
- India has skilled medical professionals and a diverse patient population. China stands out with its robust research capabilities and growing clinical trial landscape.
- South Korea and Singapore are attractive options due to their advanced healthcare systems and efficient regulatory processes.
- Australia and Japan are also preferred choices, offering high-quality research facilities and a favorable regulatory environment.
- These countries provide diverse patient pools, experienced investigators, and streamlined processes, making them destinations for successful clinical trials in the region.
The table below illustrates the countries with the application process and timelines.
| Country | Regulatory Authority | Application process (sequential or parallel) | Timeline for the approval process | Clinical trial application language |
|---|---|---|---|---|
| Japan | Pharmaceuticals and Medical Devices Agency (PMDA) | Sequential Process – Submit Clinical Trial Notification (CTN) to PMDA. The approval process involves a PMDA review and an Ethics Committee evaluation. | 30-60 days for Clinical Trial Notification (CTN) review by PMDA and Ethics Committee evaluation. | Japanese |
| South Korea | Ministry of Food and Drug Safety (MFDS) | Sequential Process – Submit an Investigational New Drug (IND) application to MFDS. Requires approval from both the MFDS and the Institutional Review Board (IRB). | 60 to 90 days for the MFDS to review the Investigational New Drug (IND) application, and for Institutional Review Board (IRB) approval. | Korean |
| Australia | Therapeutic Goods Administration (TGA) | Parallel Process – Submit Clinical Trial Notification (CTN) to the TGA and Ethics Committee simultaneously. Approval from both authorities is required. | Typically, it takes 60 days for Clinical Trial Notification (CTN) review by TGA and Ethics Committee approval, which can run concurrently. | English |
| India | Central Drugs Standard Control Organization (CDSCO) | Parallel Process – Submit the clinical trial applications to CDSCO and seek Ethics Committee approval concurrently. Both approvals are essential for starting the trial. | varied, but generally 3-6 Months for clinical trial application review by CDSCO and Ethics Committee approval, processed in parallel. | English |
| Thailand | Food and Drug Administration Thailand (FDA Thailand) | Parallel Process – Submit the Clinical Trial Application (CTA) to the FDA Thailand and the respective Ethics Committee concurrently. Approval from both is necessary. | Approximately 60 to 90 days for Clinical Trial Application (CTA) review by FDA Thailand and Ethics Committee approval, occurring in parallel. | Thai and English |
| Singapore | Health Sciences Authority (HSA) | Parallel Process – Submit a trial application to HSA and obtain Ethics Committee approval simultaneously. Require both approvals for initiation. | Roughly 60 days for clinical trial application review by HSA and Ethics Committee approval, which can be processed concurrently. | English |
| China | National Medical Products Administration (NMPA) | Sequential Process – Submit a clinical trial application to the NMPA, followed by approval from the Ethics Committee. Sequential approval is required in China. Clinical trial application language | After 60 working days, if the applicant does not receive a rejection or an inquiry for clarification from the NMPA. | Standard Chinese |
Note: The above-provided information such as approval timelines may differ from the type of clinical trial approval you would like to seek, type of research, etc. Hence it is advised to reach out to us to know what exactly process your application requires and what would be the approximate timelines for your specific application.
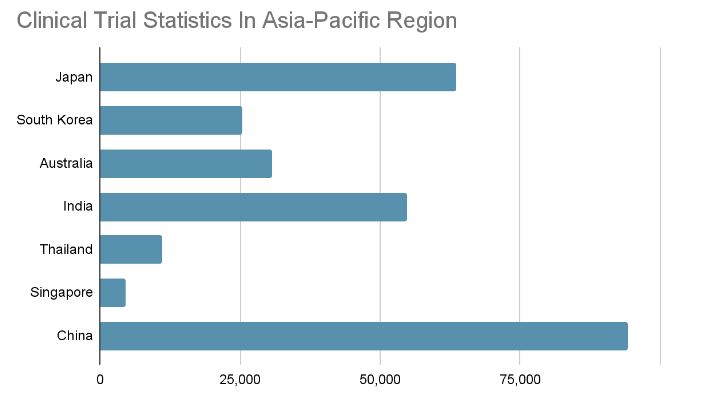
Clinical Trial Statistics in the Asia-Pacific Region
The graph below shows the latest information regarding the total number of clinical trials in the Asia-Pacific region from 1999-2022.
Best practices for successful clinical trials in Asia-Pacific
For successful clinical trials in the Asia-Pacific, collaborate closely with local experts to understand healthcare nuances. Adapt trial procedures to respect diverse cultural beliefs, ensuring participant trust. Establish effective communication channels through proper translations of materials and open dialogues with participants and regulators. These practices foster community engagement, enhance participant recruitment, and contribute to the overall success of clinical trials in the region.
Collaborating with local experts
- Establish strong partnerships with local healthcare professionals to navigate regional intricacies
- Engage local investigators early, ensuring insights into healthcare infrastructure and compliance with local regulations
- This collaboration fosters a deeper understanding of the community and enhances the trial’s credibility
Adapting to cultural beliefs
- Tailor trial procedures to align with cultural norms
- Design participant recruitment strategies that respect local customs
- Adapt informed consent processes to be culturally sensitive, fostering trust and willingness to participate
- Addressing cultural considerations promotes participant engagement and trial success
Establishing effective communication channels
- Implement clear and culturally appropriate communication channels
- Ensure the accurate translation of trial materials
- Facilitate open dialogues between investigators, participants, and regulatory authorities
- Timely and transparent communication builds trust, addresses concerns, and contributes to the overall success of the clinical trial
Conclusion
In conclusion, selecting the Asia-Pacific region for clinical trials demands strategic foresight. Collaborating with local experts, adapting to cultural nuances, and prioritizing effective communication are key. Embracing the region’s diversity ensures ethical, culturally sensitive trials. The rich patient pool and regulatory framework offer immense opportunities.
However, navigating varied landscapes requires meticulous planning and understanding. Success hinges on building trust, respecting cultural beliefs, and maintaining transparent communication. By strategically integrating these insights, researchers can unlock the potential for impactful and successful clinical trials in the dynamic and diverse Asia-Pacific landscape, contributing to advancements in global healthcare.
Do You Have Any Questions About Starting Your Clinical Trials In Asia-Pacific Regions?
Do you have any questions about conducting your clinical trials in the Asia-Pacific region or do you need our support in planning and/or executing your clinical trials in any country within the Asia-Pacific region? Please provide your requirements in the form below to connect with our expert team and further discuss your needs.