Registering Nutraceutical Products in Singapore: A Comprehensive Guide

Singapore has strict regulations on nutraceutical and dietary supplements to guarantee these health products’ safety, quality, and efficacy. It is essential for businesses intending to introduce nutraceuticals to the Singaporean market to comprehend the regulatory framework, the registration process, and the associated costs.

Let’s understand the possibilities of the Singaporean market for nutraceutical products before getting into the regulatory details.
Singapore nutraceutical market
- Anticipated to achieve 1.6 billion SGD by the end of 2023, the revenue of the Singaporean nutraceutical industry is poised for significant growth.
- The increasing demand for products targeting bone and heart health is driven by the aging population, complemented by the influence of urban lifestyles and a passion for sports.
- The nutraceutical market in Singapore is currently in its late growth stage, projected to exhibit a positive compounded annual growth rate (CAGR) of 3.4% in terms of revenue throughout the forecast period from 2018 to 2023.
- One of the fastest-aging populations in the world is found in Singapore, a factor for huge market scope.
- The amount that the government spends on healthcare is increasing; it has reached S$13.2 billion in 2020 and is expected to reach S$36 billion in 2029.
Regulatory in Singapore
Regulatory authority in Singapore
In Singapore, the oversight of nutraceuticals and dietary supplements is chiefly under the purview of the Health Sciences Authority (HSA). The HSA ensures the safety, effectiveness, and quality of health products of nutraceutical products.
Classification of products
The HSA classifies health products, such as nutraceuticals and dietary supplements, based on their ingredients, intended usage, and safety characteristics.
Depending on their risk profile, products may fall into either the
- “General Sale” or
- “Pharmacy Only
Flow for product classification
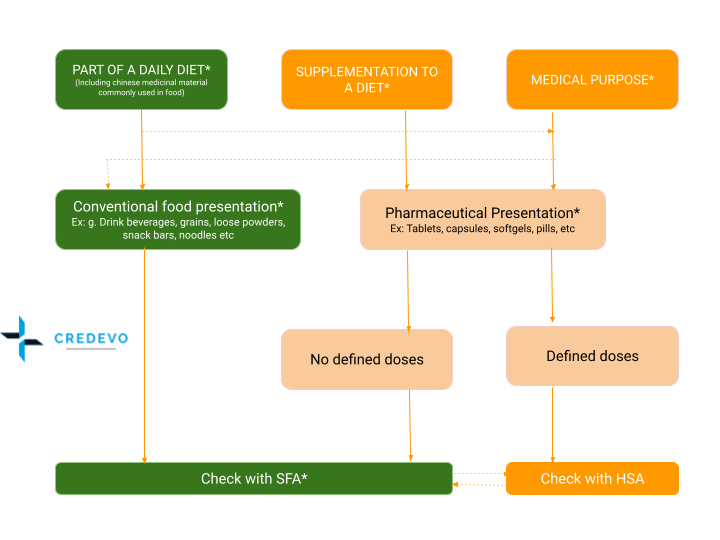
Definition of health supplement in Singapore
In Singapore, a health supplement supplements a diet and supports, maintains, enhances, and improves the healthy functions of the human body. It cannot be an injectable or a preparation that needs to be sterile, such as injections and eyedrops. It cannot be an item of a meal or diet.
A health supplement must also contain one or more, or a combination of the following ingredients:
- Vitamins, minerals, amino acids, fatty acids, enzymes, probiotics, and other bioactive substances;
- Substances derived from natural sources, including animal, mineral, and botanical materials in the forms of extracts, isolates, concentrates; and
- Synthetic sources of ingredients are mentioned in (1) and (2) points.
Health supplements belong to the group of complementary health products
Licensing & Registration
Manufacturers, importers, and distributors of nutraceuticals and dietary supplements must obtain appropriate licenses or registrations from the HSA before marketing and selling these products in Singapore.
Good Manufacturing Practices (GMP)
GMP guidelines are international quality standards that ensure the consistency, quality, and safety of the products. The HSA may conduct inspections of manufacturing facilities to verify compliance.
Health Claims
- In Singapore, companies must not label, advertise, or promote health supplements for any specific medicinal purpose.
- This includes claims that suggest treatment or prevention of any disease, disorder, or related conditions.
What documents are required for the notification process?
- Copy of the submitted form
- Manufacturer’s license/certification
- Certificate of Analysis (including appropriate test parameters, their specification, and references)
- Final artwork or product label (including the location of batch number and expiry date)
- Product leaflet and more
Yet, you may need to submit more documents based on the product class.
Fill in the form below to connect with us to know your product class and the required documents for the notification process
Notification process for nutraceuticals/food supplements in Singapore
Three simple procedures comprise the complete application process for nutraceuticals.
- Compilation
- Submission of application
- Document Submission
Let’s look at each step of the procedure in more detail below.
1. Pre-Application Preparation
- Before initiating any process of registration, it is crucial to ensure that the nutraceutical product complies with Singapore’s regulatory requirements
- Local Responsible Person (LRP): The second crucial point in the registration process is appointing an LRP who is a resident of Singapore and will liaise with the HSA on your behalf.
2. Application Submission
Voluntary notification of health supplements
- Applicants may voluntarily notify HSA of their health supplements supplied in Singapore.
- Companies must provide relevant documents to the HSA to demonstrate that their products meet the necessary safety and quality standards
- Prepare and submit the nutraceutical product registration through the HSA’s online portal.
3. Document Submission
- Furnish comprehensive details regarding your product, encompassing its formulation, ingredients, concentrations, and intended use.
- Supply information about the manufacturing facility, demonstrating adherence to Good Manufacturing Practices (GMP) standards.
- Incorporate details on quality control procedures and testing methods designed to ensure the consistent and high quality of the product.
- Substantiate the safety of your product with scientific evidence.
- If your product makes specific health claims, provide scientific evidence demonstrating its efficacy.
Nutraceutical Product Labeling & Packaging Requirements
- The HSA requires nutraceuticals and dietary supplements to adhere to precise labeling and packaging standards.
- This entails furnishing precise details regarding the product’s ingredients, dosage instructions, potential side effects, and storage conditions.
- The guidelines set by the HSA play a crucial role in guaranteeing that consumers receive transparent and accurate information about the products they acquire.
Health supplement product notification timeline & fees
Timelines
The company’s stop clock does not account for the duration taken by you to respond to our requests for clarification or additional information. The processing time for new product notification submissions is 60 working days.
- Completeness of the Application.
- Product Complexity.
- HSA Workload.
- Inspections.
- Regulatory Changes
Notification fee
There is no notification fee.
Post-Market Surveillance for Nutraceuticals
The HSA conducts ongoing monitoring of the market to identify adverse events, concerns about product safety, and issues of non-compliance concerning nutraceuticals and dietary supplements. Manufacturers and distributors must promptly notify the HSA of any adverse events linked to their products.
Do You Have Any Questions Regarding The Notification Of Your Nutraceutical Products In Singapore?
Need assistance with registering your nutraceutical/generic/pharmaceutical/medical device products, or seeking support for business expansion in Singapore? Share your requirements below to connect with our team of experts.