Cosmetics Market Entry: A Guide to Malaysia’s Notification Process

Navigating the regulatory landscape for cosmetics in Malaysia is a critical step for businesses seeking market entry. In this in-depth exploration, we delve into the intricacies of the cosmetics notification and registration process, shedding light on the key requirements set by the National Pharmaceutical Regulatory Agency (NPRA).

Our guide offers valuable insights, and step-by-step procedures to streamline the journey through the cosmetics industry’s regulatory framework, whether you’re a local entrepreneur or an international brand eyeing the vibrant Malaysian market. Join us as we demystify the process, empowering you with the knowledge to successfully bring your cosmetic products to market in Malaysia.
Malaysia Cosmetics Market
- Increasing disposable income, changing consumer lifestyles, and a growing awareness of personal grooming are driving steady growth in the cosmetics market in Malaysia.
- Key segments within the cosmetics market include skincare, haircare, makeup, fragrances, and more.
- In 2023, analysts projected the Malaysian cosmetics market to reach a value of US$436.50 million, with an anticipated annual growth rate of 3.12% (CAGR 2023-2028).
- In 2023, sales contributions from each person in Malaysia’s cosmetics business are expected to amount to US$12.72. Moreover, analysts anticipate that Non-Luxury will represent 76% of sales in Malaysia’s cosmetics market by 2023.
- the halal cosmetics sector has gained prominence in Malaysia, with consumers seeking products that comply with Islamic principles.
Top 10 reasons to choose Malaysia for your cosmetics
- Steadily growing and diverse economy.
- Sizable and diverse population with varied preferences.
- Rising disposable income supports higher spending on cosmetics.
- Growing awareness of beauty and skincare trends.
- Demand for halal-certified cosmetics is increasing.
- Membership in ASEAN provides access to a broader regional market.
- Modern retail outlets, distribution channels, and e-commerce platforms.
- Government incentives and support for business development.
- English is widely spoken, facilitating communication for international businesses.
- Tourism contributes to the demand for cosmetics.
Regulatory
The regulatory authority for cosmetics products in Malaysia is the National Pharmaceutical Regulatory Agency (NPRA), operating under the Ministry of Health (MOH). The regulatory framework ensures the safety, quality, and efficacy of cosmetic products in the Malaysian market.
Definition
According to the Guidelines for Control of Cosmetic Products in Malaysia, cosmetic products are defined as
“any substance or preparation intended to be placed in contact with various external parts of the human body (epidermis, hair system, nails, lips, and external genital organs) or with teeth and the mucous membranes of the oral cavity, with a view exclusively or mainly to cleaning them, perfuming them, changing their appearance and/or correcting body odors and/or protecting them or keeping them in good condition.”
Classification of Cosmetics in Malaysia
The classification of cosmetics products in Malaysia is generally based on the intended use and formulation of the products. The classification may include various categories, and here are common classifications.
- Categories Based on Intended Use:
- Skincare Products: Including creams, lotions, serums, and moisturizers.
- Haircare Products: Such as shampoos, conditioners, hair treatments, and styling products.
- Makeup Products: Including foundations, lipsticks, eyeshadows, and other color cosmetics.
- Fragrances: Perfumes, colognes, and scented products fall into this category.
- Personal Hygiene Products: Such as soaps, body washes, and deodorants.
- Sunscreen and Sunblock Products: Products designed for sun protection.
- Categories Based on Formulation:
- Halal Cosmetics: Products complying with Islamic dietary laws.
- Natural or Organic Cosmetics: Products made from natural or organic ingredients.
- Anti-aging Products: Products formulated to address signs of aging.
- Specialized Categories:
- Medical Cosmetics or Cosmeceuticals: Products with medical benefits, often sold in pharmacies.
- Children’s Cosmetics: Products formulated for use by children.
- Regulatory Classes:
- Notification Products: Generally, cosmetics products need to be notified to the NPRA before being placed on the market.
- Registered Products: Some products with specific claims or ingredients may require formal registration with the NPRA.
- Product-Specific Categories:
- Nail Care Products: Including nail polishes, nail strengtheners, and polish removers.
- Tattoo Products: Such as temporary tattoos and body art products.
It’s important for applicants to carefully consider the classification of their cosmetics products to ensure compliance with regulatory requirements.
Need assistance in classifying your products and not sure about the requirements to notify your cosmetic products in Malaysia? Fill out the form below to connect with our team.
Do you need a Cosmetic Notification Holder (CNH) to Notify your cosmetic products in Malaysia?
Cosmetic Notification Holders (CNH) are the companies that put cosmetic products on the market.
- The CNH ensures the availability and accessibility of information about cosmetic products, including documents related to product quality, safety, and claimed benefits, for the National Pharmaceutical Regulatory Agency (NPRA) when needed.
- The CNH must be a local company or legal entity in the cosmetics field. Also, the CNH may or may not own the products.
- This means that if foreign companies want to sell their cosmetic products in Malaysia, they don’t have to register in Malaysia. Instead, they can choose a third-party company in Malaysia to be the CNH.
The major responsibilities of a CNH include:
Responsibilities of a CNH
- Complete all transactions with the NPRA.
- Make sure the cosmetic product follows all the rules and guidelines.
- Have the Product Information File (PIF) ready with updated safety and efficacy info when asked.
- If the NPRA recalls a product, stop selling it in the market.
- Inform the health authority about any changes made to the product after it has already been sold.
Requirements for notification
Submit all documents and materials to the NPRA in either Bahasa Malaysia or English. Ensure that, when translating from other languages, you officially endorse or authorize the translations.
1. Safety Guidelines for Cosmetic Products:
- Ensuring the safety of cosmetic products involves a thorough assessment covering ingredients, chemical structure, and exposure levels.
- The NPRA sets the baseline aligned with the ASEAN Cosmetics Directive. Safety measures include careful ingredient selection, adherence to local regulations, tolerance checks, proper packaging, quality control, and prompt action in case of side effects or product changes.
2. Labelling Requirements for Cosmetic Products:
NPRA mandates clear labeling on cosmetic product packaging, including the product’s name, function, usage instructions, ingredient listing, country of manufacture, company details, batch number, and expiry date. Highlight specific precautions as well.
Additional Licenses & Certificates for Cosmetic Companies
- Apart from NPRA notification, companies may need various licenses and certificates for selling, manufacturing, importing, or wholesaling cosmetic products in Malaysia.
- These include manufacturer and wholesaler licenses, clinical trial import licenses, Good Manufacturing Practice (GMP), Good Distribution Practice (GDP), certificates of free sale, and Halal certification.
- Note that while not all are mandatory, certain certificates like GMP or GDP are prerequisites before applying for NPRA notifications, wholesaler licenses, and manufacturer licenses.
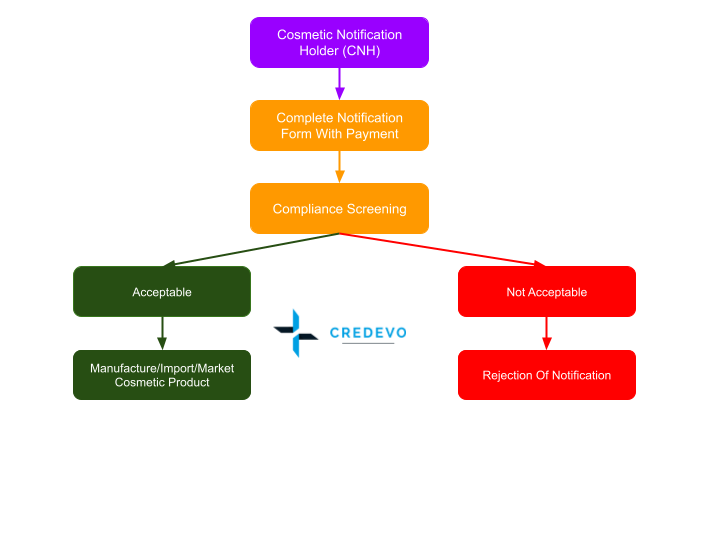
Notification process
- To begin, a CNH needs to register for a Quest membership.
- Upon successfully registering for CNH in the Quest system, the submission for cosmetic product notifications must be carried out online through the Quest system.
- The CNH is required to complete the notification form on the Quest system and pay a processing fee of Ringgit Malaysia Fifty Only (RM50.00) for each product and variant (if any) to the NPRA.
- Cosmetic products will undergo a compliance screening to assess their adherence to regulatory requirements. If they comply, a notification note will be generated, enabling the products to be registered and enter the market.
- The entire notification process may take one week to one month, depending on additional documents and information requested by NPRA.
- Notifications for cosmetic products are valid for two (years. Renewal must be done no later than one month before the notification expiry.
- The renewal fees are identical, with Ringgit Malaysia Fifty Only (RM50.00) for each product and variant (if any).
Process flow
Fee
- The notification of a cosmetic product incurs a processing fee of RM50.00 for each product (and variant, if any).
- The renewal of notification for a cosmetic product also requires a processing fee of RM50.00 for each product (and variant, if any).
Import
- Upon receiving authorization in the Notification Note from the DPS, CNH can proceed to manufacture or import the cosmetic product.
- After NPRA confirms payment and all notification requirements are met, CNH can immediately generate the Notification Note from the Quest system.
Notification validity
The validity of a cosmetic product notification lasts for 2 years. Renewal must be completed no later than 1 month before the notification expires.
Certificate of Free Sale
- Purpose and Issuance of Certificate of Free Sale (CFS):
- The Certificate of Free Sale (CFS) functions as a document indicating that the product is approved for unrestricted sale in Malaysia.
- NPRA issues the certificate upon request by the CNH, which aims to export its notified cosmetic product to a country mandating the certificate.
- Application Process for CFS:
- CNH must apply for the CFS through online submission using the Quest system.
- A fee of RM50.00 per certificate copy is charged for each application.
- Combining CFS for Products and Variants:
- CFS can cover both the main product and its variant within a single certificate.
- This consolidation is permissible if the variant is officially notified as part of the main product.
Need our support to notify your cosmetic products in Malaysia?
Talk to us today. Provide preliminary details below and we will help you achieve a successful notification in Malaysia. Credevo can be your strategic partner to make a successful submission in a timely manner. You will get a complete overview of the regulatory framework, process overview, and timelines associated with submissions.