Medical Device Registration In Thailand: Regulatory & Approval Process

The Medical Device Control Division (MDCO) from the office of the Food and Drug Administration (FDA) in Thailand (also known as the Thai FDA) oversees Medical device regulations. Recently the Thai FDA has made many changes in regulatory requirements and the approval process for medical devices.
This article was last updated in March, 2023.

Medical device market
Thailand’s medical device market has been steadily growing in recent years and is expected to continue to grow in the future. The market is primarily driven by an aging population, rising healthcare expenditures, and increasing demand for advanced medical technologies.
According to a report by the Thai Medical Device Technology Industry Association, the medical device market in Thailand was valued at approximately $2.1 billion in 2019 and is projected to reach $3 billion by 2024, with a compound annual growth rate (CAGR) of 7.4%.
Reasons for the Increase in demand for medical devices in Thailand
- Aging population: Thailand has a rapidly aging population, with the number of people over 60 expected to reach 20% of the population by 2020. This demographic shift has led to an increase in demand for medical devices, such as hearing aids, joint replacements, and pacemakers.
- Rising healthcare expenditures: The Thai government has been increasing its healthcare budget in recent years, leading to an increase in demand for medical devices and equipment. This has allowed hospitals and clinics to upgrade their facilities and purchase the latest medical technologies.
- Increasing demand for advanced medical technologies: As the Thai healthcare system becomes more advanced, there is an increasing demand for advanced medical technologies such as robotic surgery, telemedicine, and digital health solutions.
- Government support: The Thai government has implemented policies to promote investment in the medical device industry and encourage the production of medical devices in Thailand. This has led to an increase in the number of local manufacturers and suppliers, as well as an increase in foreign investment.
- Medical tourism: Thailand has become a hub for medical tourism in Southeast Asia, with many international patients coming to Thailand for medical treatments. This has led to an increase in demand for high-quality medical devices and equipment.
Regulatory authority for medical devices in Thailand
The Medical Device Control Division (MDCD) of the Thai Food and Drug Administration (FDA) oversees medical device regulations in Thailand.
Thai FDA recently released new regulations effective from February 15, 2021. The manufacturers or sponsors who obtained medical device approval under the old regulations shall renew approval as per the new regulations.
Are you looking for the regulatory & approval process in Thailand? Click here to learn
Medical device definition in Thailand
Products that make therapeutic, medical, or dental claims are considered medical devices in Thailand, including aesthetic devices and software as medical devices. The manufacturers of Medical devices with a drug component need to register as a pharmaceutical.
Medical device classification in Thailand
Based on risk and ASEAN MDD guidance, medical devices are classified into four categories. Class I, Class II, Class III, and Class IV.
| Category | Risk | Regulatory process |
| Class I | Low-risk | Listing (self-declaration) |
| Class II | Low to Moderate-risk | Notification (CSDT) |
| Class III | Moderate to High-risk | Notification (CSDT) |
| Class IV | High-risk | License (CSDT) |
Similarly, In Thailand, the FDA classifies in-vitro diagnostic devices (IVDs) into four categories based on the risk and close adherence to ASEAN MDD guidance is a standard practice.
Conformity assessment
- To market and import a medical device into Thailand, Medical devices need to undergo one of three registration routes (as given in the above table) to acquire an Import License depending on their classification.
- Class-I medical device requires less documentation.
- The low-risk Class-I devices must be listed before they are imported and marketed in Thailand,
- The Class I sterile and measuring devices require the submission of testing reports for placing these devices on the market.
- Whereas the Class II and Class III devices have to be notified and the Class IV devices must have an approved license to be placed in the Thai market.
- The Class II, III, and IV devices require submission of a technical dossier, as per ASEAN CSDT format. Also, Class II, III, & IV devices require ISO 13485:2016 certificates to certify the quality system of the legal and/or actual manufacturing facilities.
Requirements for registration of medical device in Thailand
- The language for the submission could be English.
- Dossier requirements
- Device description,
- Pre-clinical studies,
- Device labeling,
- Instructions for use,
- Risk analysis and any existing regulatory approvals or market authorizations, and
- Manufacturer compliance for ISO 13485.
- Some medical devices require separate Thai FDA permits or registrations from other Thai government agencies, such as those which contain or transmit radiation and those with wireless connectivity, and
- Some specific medical products may need local testing in an accredited laboratory in Thailand.
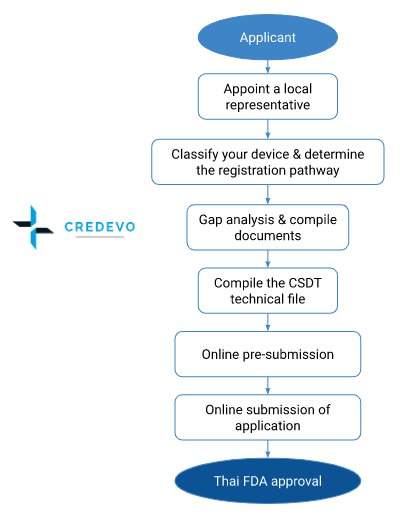
Steps to register medical devices with the Thai FDA
- To register a medical device in Thailand, a company must first obtain an Establishment License for the manufacture or import of the medical device.
- The sponsor can apply for Establishment License via manual submission and the Thai FDA issues it.
- Currently, only people of Thai nationality or locally established companies can apply for an Establishment License.
- Foreign manufacturers have three possibilities to register their medical devices in Thailand:
- Set up a legal subsidiary entity in Thailand, or
- Appoint a local distributor, or
- Appoint an independent third party.
- The holder of an Importer License may then proceed to register the medical device with the Thai FDA.
- Applicants can submit all medical device registrations in Thailand online via the Pre-submission and E-submission system.
- Once the applicant submits the required documentation in the E-submission system according to the product’s class, an order of payment will be generated. The registrant has to process payment for the application.
- After receiving the payment, the Thai FDA will evaluate the documents.
- If all documents meet Thailand’s medical device registration requirements, then the Thai FDA will issue the
- Listing Certificate,
- Notification Certificate or
- License.
- However, if the Thai FDA considers that the documentation does not meet the requirements, then requests additional documentation.
- The Thai FDA evaluates the submission once again. If the review is successful will provide authorization, or if not, it may reject the application.
Approval process flow
Labeling requirement for medical devices in Thailand
- Section 44, of the Medical Device Act of 2008 describes the regulations for labeling requirements in Thailand.
- The display of labels and medical device documentation shall be in accordance with the rules, procedures, and conditions prescribed in the Notification of the Minister.
- A seller of medical devices shall ensure the presence of labels or labels as well as medical device documentation, as the case may be, as provided by the establishment registrant, the permission grantee, the specification declarer, or the notifier under paragraph one.
Validity of medical device approval
- The approval of all medical devices is valid for five years.
- The approval holders need to renew before the expiration date with partial applications.
- If the renewal date of the approval expires, the holder shall follow the complete registration process. Documentation requirements vary for partial applications depending on the renewal date.
Review fee and timelines
The fee and timelines vary depending on the medical device classification. Below is the table which lists the fee and approval timelines.
| Risk Classification | Registration Type | Approval Fee | Max Review Time* |
| Class I | Listing | 3,100 Bhat | 200 days |
| Class II | Notification | 31,000 Bhat | 250 days |
| Class III | Notification | 31,000 Bhat | 250 days |
| Class IV | Licensing | 51,000 Bhat | 300 days |
Medical devices with wireless technology capabilities
- Medical devices that use wireless technology such as cellular, WiFi, or Bluetooth are subject to regulatory review by the Office of The National Broadcasting and Telecommunications (MBTC).
- It takes 30 to 45 working days for the government to review, and the costs involved in processing the application would be approximately US$200.
- The sponsor can obtain a license for this wireless technology before, during, or after medical device licensing; but must be in hand prior to importation.
Local importer
- A foreign manufacturer shall appoint a local importer to import medical devices into Thailand,
- The Thai FDA issues Import licenses to a local, licensed company that will own and control the license.
- A license holder also requires an establishment license issued by the Thai FDA.
Looking for regulatory support for your medical device in Thailand
Talk to us today. Provide preliminary details below, and we will help you achieve a successful registration in Thailand. Credevo can be your strategic partner to make a successful submission promptly. You will get a complete overview of the regulatory framework, process overview, and submission timelines.