Clinical Trial Sites For Covid 19 Trials
Covid 19 has caused severe disruptions for many people. According to WHO, as of 31 December 2020, COVID-19 had infected over 82 million people and killed more than 1.8 million worldwide. This pandemic raised the interest of many researchers to find the drug and treatment plans for COVID-19. So, now it’s interesting to know the impact of COVID-19 on the clinical trial/research landscape and the difficulty of finding clinical trials sites for researchers to conduct clinical trials for COVID-19.
Covid-19 is an infectious disease and the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus strain causes this infectious disease. This virus causes illnesses ranging from common flu to severe respiratory diseases and can even prove fatal too.
The first case was found in Wuhan, China, in December 2019, and during the initial outbreak in Wuhan, the virus and disease were commonly referred to as “coronavirus” and “Wuhan coronavirus”. Later, the WHO released the official names COVID-19 and SARS-CoV-2 on 11 February 2020.
A successful trial can lead to the development of new drugs intended for human use. Clinical trials have been a long and traditional method of testing and validating new medicines and therapies.
There are a number of new interventions on their way for Covid-19 with different therapies like studies of Vaccines, Medical Devices, and Medicines. However, most of the studies are in the clinical trial phases.
How coronavirus attacks the human body?
- The viruses contain spike proteins that bind to receptors on healthy cells, especially those in the lungs. This conqueror makes copies of itself and spreads all over the body.
- The lungs are the organs most affected by COVID-19 because the virus accesses host cells via the receptor for the enzyme angiotensin-converting enzyme 2 (ACE2), which is most abundant on the surface of type II alveolar cells of the lungs.
- Once it enters inside the cells, the coronavirus hijacks healthy cells and takes command. And eventually, it kills some of the healthy cells.
- COVID-19 affects the upper respiratory tract (sinuses, nose, and throat) and the lower respiratory tract (windpipe and lungs).
- It strikes the heart, incapacitating its muscles and disrupting its critical rhythm. It affects kidneys so badly that some hospitals have run short of dialysis equipment.
- Creeps along with the nervous system destroy taste and smell and occasionally reache the brain.
- Creates blood clots with sudden efficiency and inflames blood vessels throughout the body.
- Experts are working to understand more about short and long-term health effects associated with COVID-19, who gets them, and why.
“No one is safe until everyone is safe”
by WHO
Let’s better understand about symptoms, effects, and treatments of Covid-19
Symptoms of COVID-19 (Corona Virus)
The COVID-19 symptoms are variable and range from mild symptoms to severe illness.
After infection, these symptoms last weeks or months.
The common symptoms of COVID-19 include
- Headache,
- Loss of smell and taste,
- Nasal congestion and runny nose,
- Cough,
- Muscle pain,
- Sore throat,
- Fever,
- Diarrhea, and
- Breathing difficulties.
Symptoms may change over time, and people with the same infection may have different symptoms.
Effects of COVID-19 on organs
People who had a severe illness with COVID-19 experience effects on multiple organs over a long time and these symptoms may last for several weeks or months after COVID-19 illness.
- The virus can affect multiple organs, if not all, body systems, which include heart, lung, kidney, skin, and brain functions.
- Autoimmune conditions occur when the immune system attacks healthy cells in one’s owns body by mistake, and causes inflammation (painful swelling) or tissue damage in the affected parts of the body.
- While in some people, mostly children, experience multisystem inflammatory syndrome (MIS) during or immediately after a COVID-19 infection.
- MIS is a condition where different body parts can become inflamed.
- It can lead to post-COVID conditions if a person continues to experience multiorgan effects or other symptoms.
Diagnosis of COVID-19
COVID-19 is usually diagnosed based on signs, symptoms and is confirmed using Reverse transcription-polymerase chain reaction (RT-PCR) or another nucleic acid testing of infected secretions.
Treatments for COVID-19
To date, there is no specific or effective treatment or cure for coronavirus disease 2019, the disease caused by the SARS-CoV-2 virus. In cases where the disease is mild, supportive care includes medication such as paracetamol or NSAIDs in order to relieve symptoms (fever, body aches, cough), proper intake of fluids, rest, and nasal breathing.
In patients where condition is moderate to severe, may need treatment in the hospital. And, in patients with low oxygen levels, the physicians recommend the use of glucocorticoid dexamethasone.
Dexamethasone
- Dexamethasone and other corticosteroids (prednisone, methylprednisolone) are potent anti-inflammatory drugs.
- The drug is inexpensive and is readily available.
- In some cases, the immune system reacts towards treatment which damages the lungs and other organs and often leads to death.
- However, this treatment did not have a benefit in patients who did not need respiratory support.
- The results of RECOVERY TRIAL recommended this treatment
Monoclonal antibodies & Tocilizumab
- Physicians use monoclonal antibodies in combination with corticosteroids, such as dexamethasone in order to suppress the immune response in very ill hospitalized patients.
- Some COVID patients get sicker because of an overreaction of the body’s immune response (a cytokine storm) to the viral infection. And when this happens, the body overproduces interleukin-6 (IL-6), a protein involved in inflammation in lung cells.
- The tocilizumab (Actemra) received Emergency Use Authorization (EUA) grant by the US FDA, a monoclonal antibody that blocks the action of IL-6, and thereby suppresses the exaggerated immune system response.
- However, this treatment dint receive authorization for use by non-hospitalized patients with COVID-19.
Remdesivir
- Remdesivir may modestly speed up the recovery time.
- Baricitinib in combination with remdesivir benefits hospitalized adults and two years and older children who require respiratory support.
- However, there is not yet enough evidence to support the use of this therapy.
Anticoagulation drugs (“blood thinners”)
- Almost all hospital admitted patients with COVID receive medications to help prevent blood clots.
- Doctors usually prescribe low-dose heparin or enoxaparin.
Let’s understand some facts about vaccines and how they prevent covid
How do vaccines prevent covid?
In order to offer protection, different types of vaccines work in different ways. But with all types of vaccines, the body is left with a supply of “memory” T-lymphocytes as well as B-lymphocytes that will remember how to fight the virus in the future.
- It typically takes a few weeks after vaccination for the body to produce T-lymphocytes and B-lymphocytes.
- Therefore, it is possible that a person could be infected with the virus that causes COVID-19 just before or just after vaccination and then gets sick because the vaccine did not have enough time to provide protection.
- Sometimes after vaccination, the process of building immunity can cause symptoms, such as fever, headache, swelling, and pain. These symptoms are normal and are signs that the body is building immunity.
According to WHO, as of December 2020, there are over 200 vaccines for COVID-19. And of these, at least 52 candidate vaccines are in human trials. Several vaccine candidates are currently in phase I/II, which will enter phase III in the coming months
Types of vaccines
Currently, researchers follow three main approaches to design a vaccine and these three approaches are
whether they
- use a whole virus or bacterium; or
- just the parts of the germ that triggers the immune system; or
- just the genetic material that provides the instructions for making specific proteins and not the whole virus.
COVID-19 mRNA Vaccines
- The spike protein found on the surface of the virus causes COVID-19. The COVID-19 mRNA vaccines give instructions for cells to make a harmless piece of what is called the “spike protein.”
- First, the COVID-19 mRNA vaccine is given on the upper arm muscle. Once the mRNA is inside the muscle cells, they use them to make the protein piece. After making the protein piece, the cell breaks down the instructions and gets rid of them.
- Next, the cell displays the protein piece on its surface. Our immune systems recognize that the protein doesn’t belong there and begin building an immune response and makes antibodies, like what happens in natural infection against COVID-19.
- At the end of the process, our bodies have learned how to protect against future infections.
- The benefit of mRNA vaccines, like all vaccines, is that those vaccinated gain this protection without ever having to risk the consequences of getting sick with COVID-19.
Viral Vector COVID-19 Vaccines
Viral vector vaccines use a modified version of a different virus (the vector) to deliver critical instructions to our cells.
- First, the vector (not the virus that causes COVID-19, but a different, harmless virus) will enter a cell in our body and then use the cell’s machinery to produce a harmless piece of the virus that causes COVID-19. This piece is known as a spike protein only found on the surface of the COVID-19 virus.
- Next, the cell displays the spike protein on its surface, and our immune system recognizes it doesn’t belong there. This triggers our immune system to produce antibodies and activates other immune cells to fight off what it thinks is an infection.
- At the end of the process, our bodies have learned how to protect us against future infection with the virus that causes COVID-19.
- The benefit is that we get this protection from a vaccine, without ever having to risk the serious consequences of getting sick with COVID-19.
- Any temporary discomfort experienced after getting the vaccine is a natural part of the process and an indication that the vaccine is working.
Inactivated vaccine
- The first way to make a vaccine is to take the disease-carrying virus or bacterium, or one very similar to it, and inactivate or kill it using chemicals, heat, or radiation.
- This approach uses technology that’s been proven to work in people and this is the way the flu and polio vaccines are made.
- However, it requires special laboratory facilities to grow the virus or bacterium safely, can have a relatively long production time, and will likely require two or three doses to be administered.
Now we move on to trial-related information is based on clinicaltrials.gov.
Impact of Covid-19 on clinical trials
Covid a disruption in clinical trials
- Around 80% of non-COVID-19 trials were stopped or interrupted as a result of the COVID-19 pandemic.
- Recent FDA guidance recommends companies who conduct clinical trials to consider virtual patient visits or put new processes in place regarding their current protocols.
- Shutdowns were most frequently driven by concerns that patients would not be able to safely travel to and receive care at research centers.
- Due to covid-19 and imposing of strict lockdown, there is a direct and huge impact on on-site activation and in the continuity of running trials.
- The US has the highest number of disrupted trial sites (66.1%, while the UK has the disrupted site 12.8%, France, Spain and Germany have 9.3%, 6.5%, and 5.2% respectively.
Sponsors, CROs, and other organizations that work for drug development were forced to shift to remote working environments.
Clinical trials on Covid-19
Several experimental treatments are being actively studied in clinical trials. Despite ongoing research, there is still not enough high-quality evidence to recommend so-called early treatment.
Some interesting facts for clinical trials on Covid-19.
- Do you know, according to clinicaltrials.gov, a total of 6,518 clinical trials were registered for Covid 19?
- The data includes all conditions that are relevant to Covid-19 such as Pneumonia, Respiratory Infection, Acute Respiratory Distress Syndrome, Acute Bronchitis, and Covid-19 Lower Respiratory Infection. Different trial designs show how the clinical trials have been carried out.
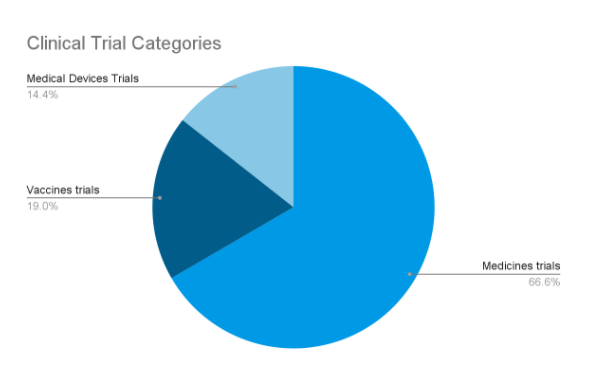
- The data which is extracted from clinicaltrials.gov has been studied for three different categories for medical devices, vaccines, and medicines.
- The medical devices studies are 697 in number,
- Vaccines studies are 918 in number, and
- Medicines with the highest number of studies is 3,224 in number (as shown in pie graph)
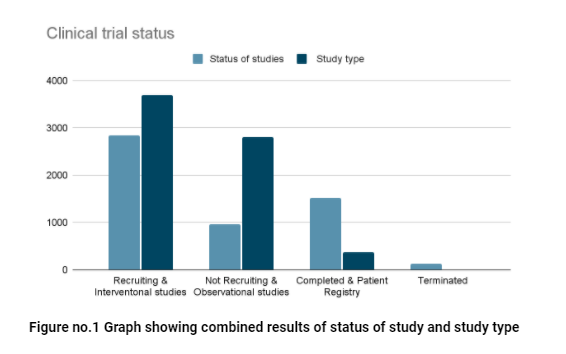
- This data enables us to identify the various factors like data of the status of studies, what the study type is (well explained in figure no.2), and the type of funding for the study.
- Status of study data include the stage of study,
- Getting approval but still in process to start recruiting,
- Whether they are recruiting the subjects,
- Completed number of studies and the terminated number of studies.
Geographical Location
- The highest number of clinical trials are conducted in the United States and the number is 1,465.
- 798 studies are registered in France.
- China has recorded 225 clinical trials.
- Germany and Italy have recorded 201 and 328 trials.
- Switzerland has 93 trials.
- India has recorded 95 studies overall, in which 66 studies are going on medicines trials, 8 medical devices trials and vaccines have 18 trials.
Now, moving forward with the trial design study types having two types are
Interventional Studies
- These are the studies in which participants are assigned to groups that receive one or more treatments. So, researchers can evaluate the effects of interventions on biomedical health.
- Data on the clinical trials site shows that Covid-19 has 3,685 Interventional studies.
Observational Studies
- Observational studies aim to find out what happens to people in different situations.
- The research team observes the people taking part, but they don’t influence what treatments people have.
- 2,799 studies were observational studies in clinical rials for Covid-19.
Clinical trial sites for research on COVID treatment
It is very challenging to find good sites in a short duration to conduct clinical trials. However, the site selection efficiency can be improved by having a checklist of criteria for good sites selection.
Here are few tips to select sites in simple steps
Step 1: Define site requirements and selection criteria
- Facilities and Equipment
- Staff qualifications
- Site profile and timelines
- Past performance
- Population profile and access
You can assess this easily by conducting a feasibility study.
Click here to learn about clinical trial feasibility
Step 2: Identify sites and gather initial information
- Clinicaltrials.gov postings
- Publications of recent clinical trials in the studied indication
- A site network organization that owns a network of sites, oftentimes with various experience
Step 3: Evaluate and select sites
- Provide a standard budget template to the sites as this will ensure that all items are accounted for and facilitate budget comparison.
- The site monitors then need to conduct Pre Study Visits (PSVs), also called Site Selection Visits (SVSs) or Site Assessment Visits (SAVs), especially if the site is new to the sponsor or indication, or if key site staff has changed since their last participating trial.
Do you need support for your COVID 19 clinical trials?
Discuss your clinical trial needs with us. We will help you in developing a strategy for an effective and efficient clinical development pathway.