Importation of Clinical Ancillary Supplies for Clinical Trials in China

As clinical trials have become increasingly global and complex, ancillaries have grown in volume, type, and expectations. The clinical trials supply market is projected to reach USD 2.5 billion by 2025 owing to the logistics services with the increasing number of clinical trials involving temperature-sensitive drugs. Currently, North America is the largest market for clinical trial supplies while China dominates the Asia-Pacific clinical trial supplies market.

Before moving into further details, let’s understand first what are clinical ancillary supplies.
Clinical ancillary supplies
Clinical trials require a wide variety of ancillary supplies such as diagnostic, medical, laboratory, drug delivery equipment, instruments, consumables, and other general supplies.
Some examples of commonly used ancillaries include refrigerators, centrifuges, computers, ECG machines, and glucose meters, as well as consumables such as gloves, alcohol swabs, face masks, and test strips. Trials of diabetes, vaccine, cardiovascular, and oncology generally require a high level of ancillary products.
A clinical trial cannot begin unless both the Investigational Medicinal Products and ancillary components are in place. Ancillaries have become more sophisticated as sponsors embrace patient-focused treatments and precision medicine. Hence, sponsors should equally focus their attention on supplying ancillaries in clinical trials.
Looking to conduct clinical trials in China? Click here.
Top challenges related to the ancillary supplies of clinical trials
- Varying regulatory guidelines and timelines between the countries.
- Ensuring that ancillary supplies reach sites on time and in a cost-efficient manner.
- Managing the procurement, storage, and distribution.
- Import/export challenges.
Some of the key considerations and better planning of ancillary supplies
- Plan for import of ancillaries unless they are not available locally.
- For importing, ensuring that you have approved ancillaries for every country.
- Ancillary management at every stage of the clinical trial life cycle.
- Planning for IMPs and ancillaries at the same time, especially before making a clinical trial application.
- Identify the key partners who can efficiently handle your importation process.
Let’s see the scenario of the importation of clinical ancillary supplies in China and the challenges involved.
Import of clinical ancillary supplies in China
Along with global ancillary supplies challenges, the importation of clinical trials supplies in China is a little more critical and present some unique challenges such as
- Language barrier,
- Changing regulations,
- Varying requirements for each ancillary product,
- Unpredictable import process timelines, and
- Stringent custom inspections.
The process to import clinical trial supplies in China
Given the various rules and regulations, and different requirements for each category, one may wonder how to start the process of importation of clinical trial supplies in China.
The process of importation can be summarized in five stages, as follows.
- Understand the regulations.
- Obtain Clinical Trial Approval.
- Collect the necessary documents required for importation.
- CIQ and Customs Clearance.
- Depot and Distribution.
Credevo experts have reviewed all these details and found answers to all such questions. Based on such review, a report with information on the complete process import of clinical ancillary supplies has been prepared. This ‘Report on the Process for Import of Clinical Ancillary Supplies into China‘, provided by Credevo, can be downloaded by clicking the below details.

To make a successful importation you need to answer the following questions related to the import of clinical ancillary supplies into China.
- What are the regulations for the import of ancillary supplies in China?
- Do we need to classify the ancillary products? If yes, how are ancillary products classified?
- What are the certifications and approvals required for various ancillary products?
- How can an Importer of Record (IOR) help you with such importation?
- Do we need to have a clinical trial approved before importation?
- What are the essential formalities to be made before making an importation?
- How about the CIQ and custom clearance?
- What is the process during and after importation?
- What about the port of import, depot, and distribution consideration?
Get details on the Regulatory Process, Requirements, and Aspects of Importation of Clinical Trial Ancillary Supplies by downloading this report.
Credevo has prepared this report by collecting the relevant information as per the current understanding and awareness of Credevo. Click on the blue button below to download the report.
Note: The details presented in the report are intended to help in creating awareness about the regulatory process and requirements for importing Clinical Ancillary Supplies into China. While the report can be considered as an informative resource, it is not being presented as legal guidance, a binding document, or any authoritative report. The information in this report was prepared on the basis of relevant information from the public domain as of the date, however, the Chinese regulations and requirements may change with time and it is advisable to refer to current regulations at any given time. It is recommended to confirm the specific details, requirements, and processes applicable to your specific products at a given time before initiating any process of importation.
Stage 1: Understand the regulatory
Before initiating any process for importation, identify the regulations that apply for each product and unique customs requirements based on the HS code. Different categories have different import requirements as follows
- Import license
- Registration certificate for medical devices
- Certificate For China Compulsory Product Certification (CCC)
- China Inspection and Quarantine (CIQ)
Import requirements on the basis of HS code can be obtained by seeking a pre-classification opinion from third-party service providers designated by Chinese customs.
Stage 2: Obtain Clinical Trial Approval
The sponsor needs to submit a Clinical trial application to NMPA. If the applicant does not receive any rejection or inquiry, it takes 60 working days for approval, If the application does not meet the requirements additional information must be submitted by the applicant within five days of CDE’s notice.
After getting approval, the process for obtaining certifications and documents can be initiated as per the HS code to facilitate the import process.
Stage 3: Collect the necessary documents required for importation
The sponsor should initiate the process of obtaining the following necessary documents based on the HS code.
Import license
Import license regulation in China is used to keep control of imported products and import quotas. An importer needs to obtain the import license before the shipment is made.
The products that need the import license are broadly classified as
- Permitted goods
- Permitted goods that need to be monitored
- Restricted goods
- Prohibited goods
You need to clearly identify which applicable products require the import license.
Registration certificate for medical devices
Medical Device Registration Certificate (MDRC) is issued by NMPA. It is generally mandatory for the import of all the medical ancillaries. Products must have a country of origin approval before applying for registration in China.
Certificate For China Compulsory Product Certification (CCC)
CCC is a compulsory safety mark certification required to import some products in China. CNCA (Certification and Accreditation Administration of China), is the agency that approves the CCC mark. The product samples are tested in the accredited Chinese testing laboratories before getting a CCC mark.
CIQ import license
China Inspection and Quarantine import license is required to meet the Chinese standards for eligibility for import into China. It is useful to check for the applicability of the import license during the initial confirmation of requirements as per the HS code.
Customs Declaration
The customs declaration form is required to be submitted at the customs for clearance of imported supplies. The details such as customs registration code (CR Code) number, the port of import, etc need to be provided while submitting a customs declaration form.
Stage 4: CIQ and Customs Clearance
CIQ inspection is conducted after shipment arrival into China.
China Inspection and Quarantine
The imported products undergo inspections, preliminary CIQ inspection followed by the customs authorities. Some of the tasks performed by the CIQ are
- To inspect and control goods on arrival,
- Check for labeling and packaging of products,
- Make sure that products have CCC marking and stated as before,
- Check all applicable certifications required for import, and
- Check whether the exporters follow the local Chinese regulations.
The products need to pass a quarantine inspection before they move for customs clearance.
If the goods do not pass the CIQ inspection, they are sent back or kept at the port for several days. Submitting a Declaration to CIQ before reaching port avoids delays. Once the goods have passed the CIQ inspection, are then transferred to customs clearance.
Custom Clearance
Attention to detail is required as the packing list covers specific information such as the number of pieces, net weight, etc. The timeline for the customs clearance may take from a few hours to several days. It can be tracked by using the declaration number and verification code.
After a successful customs clearance, facilitating depot is mandatory in China.
Stage 5: Depot and distribution
After the customs clearance, the importer recovers the shipment and transfers it to the depot. Goods at the deport are inspected for labeling and packing conditions. Once the inspection is complete, the shipments are distributed to the sites located all over China.
Process flow for import
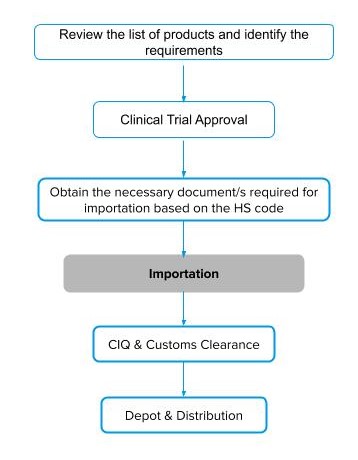
Figure 1: Flow of import process
Who is an Importer of Record (IOR) and Why having an IOR is necessary for China?
An Importer Of Record (IOR) is a legal entity or an organization that performs certain duties on behalf of the importer. The Importer of Record can be particularly confusing as it varies from country to country. The process of IOR services starts with making a legal contract with the client.
The importer of record in China gathers all the documents necessary for importing goods and provides them to Chinese Customs agents. All importers in China are required to have Importer Customs Registration Codes (CR code).
References
- https://www.fisherclinicalservices.com/content/dam/FisherClinicalServices/Learning%20Centre%20Images/Resources/eBook%20Images/Ancillary_Management__Keep_Your_Clinical_Trial_On_Track.PDF
- https://www.parexel.com/solutions/clinical-development-services/clinical-trial-logistics/ancillary-supplies
- https://www.inceptua.com/wp-content/uploads/2019/07/White-Paper-Navigating-the-clinical-trial-supply-route-in-china_2019.pdf
- https://www.fisherclinicalservices.com/content/dam/FisherClinicalServices/Learning%20Centre%20Images/Resources/Latest%20Article%20Images/Ancillary_Supplies_Article.pdf
- https://www.grandviewresearch.com/industry-analysis/clinical-trial-supplies-market