Rare Diseases: Registration & FDA Support For Orphan Drugs

Orphan drug designation for rare disease qualifies the sponsor of the drug for various development incentives of the ODA, including tax credits for qualified clinical testing, so let’s know more about Orphan drug designation

Worldwide orphan drug sales are forecast to grow at a CAGR of 12.3% from 2019 to 2024, which is approximately double the rate forecast for the non-orphan drug market. By 2024, orphan drugs are expected to reach $242bn and capture one-fifth of worldwide prescription sales as per a report.
The US Food and Drug Administration (FDA) in 2019, has approved an increasing number of orphan drugs for rare disease and biologics, as well as drugs targeted at specific cancers. The Center for Drug Evaluation and Research (CDER) has reported that this value was more than twice compared to the past eight years.
What is an orphan drug and orphan drug designation for rare disease?
Orphan drugs are medicinal products intended for the diagnosis, prevention, or treatment of life-threatening or very serious diseases or rare disorders where human consumption may be very few.
A special status is granted to a drug or biological product to treat a rare disease or condition upon request of a sponsor. as per the Orphan Drug Act (ODA) and this status is referred to as orphan designation (or sometimes orphan status).
The main goal of orphan drug regulation is to encourage pharmaceutical companies to develop the process of research and production of orphan drugs.
Key points to consider for an orphan drug designation
- It is wise to seek orphan drug designation early stage of the drug development process for rare diseases to obtain incentives offered during drug development.
- Once an orphan medicine is authorized, it is provided with 7 years of market exclusivity and the market exclusivity becomes effective once the Manufacturing Authorisation (MA) is granted.
- The granting of an orphan designation request may not alter the standard regulatory requirements and process for obtaining marketing approval.
- The safety and effectiveness of a drug must be established through adequate and well-controlled studies.
How can a sponsor apply for an orphan drug designation?
A request for orphan drug designation can be submitted electronically on a single compact disk (CD) with a signed cover letter attached and preferred to be sent by regular or express post. An orphan drug designation request cannot be submitted by email or by any other physical media.
Review and approval process for a rare disease drug to receive orphan drug designation
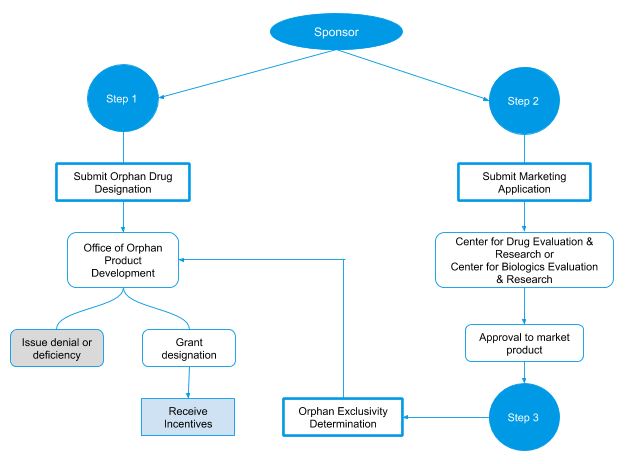
- The application receives a designation request number, log into the OOPD database, and an acknowledgment letter will be sent to the sponsor (or sponsor’s agent).
- An Office of Orphan Products Development (OOPD) reviews the request, which may require consultation with an FDA Center.
- The review is forwarded to the Director of the Orphan Drug Designation Program for a second-level review.
- Following the OOPD Office Director’sreview, a designation letter, or a deficiency letter requesting additional information, or a denial letter is prepared and issued to the sponsor.
FDA requirements for orphan drug designation
- Copies of all materials documenting how the prevalence estimate was made should be provided in the designation request and if the reference source is from a website/s, website/s address along with a hard copy of the document should be included.
- Prevalence of a rare disease can be found on the internet at government and patient support group websites, references, and literature.
- Preferring the most recent data and involving the U.S population should be considered. Proper justification shall be included if using foreign data (how this can relate to U.S population data), or old data
- Drugs for an acute condition (i.e. less than one-year duration) incidence may be used as an estimate of the population and if the disease is relapsing/remitting, a prevalence estimate may still be required.
- For the drug which is intended for diagnosis or prevention of a rare disease or condition, the estimated number of people to whom the drug will be administered annually shall be submitted.
For a drug to qualify for orphan designation both the drug and the disease or condition must meet certain criteria specified in the ODA and FDA’s implementing regulations at 21 CFR Part 316.
Process for orphan designation
How can a foreign sponsor apply for orphan drug designation?
A U.S. permanent resident is required as an agent to file a request for an orphan drug designation for a foreign sponsor and is responsible for the paperwork involved with the designation request and if a designation is granted, it will serve as the contact person afterward.
OOPD makes all the correspondence with the U.S.permanent-resident agent and this includes submitting subsequent annual reports after a product is designated.
What are the incentives or benefits offered by the FDA for orphan drugs in rare disease?
Yes, orphan designation qualifies the sponsor of the drug for various development incentives of the ODA, including tax credits for qualified clinical testing.
Learn more about various incentives provided for drug development in rare diseases by regulators in Europe, Australia, Japan, Russia, South Korea, India & China
- Financial
- Tax incentives: The Orphan Drug Tax Credit (ODTC): sponsors who have an orphan designation can collect tax credits for expenses incurred after the issue of the designation for U.S. clinical trial costs on the orphan indication.
- User fee: Orphan drugs and products are exempt from the usual new drug application or “user” fees charged by the FDA.
- Regulatory benefits
- The Rare Pediatric Disease Priority Review Voucher Program says that a sponsor who receives approval for a drug or biologic for a “rare pediatric disease” may qualify for a voucher that can be redeemed to receive a priority review of a subsequent marketing application for a different product.
- A fast-track procedure for the FDA to evaluate registration files.
- Eligible to receive regulatory assistance and guidance from the FDA in the design of an overall drug development plan.
- Clinical development benefits
- Orphan Product Grant program provides funding for clinical testing of new therapies to treat and/or diagnose rare diseases that lower the cost of drug development.
- Medical device
- The Humanitarian Use Device (HUD) program designates medical devices that are intended to benefit patients in the treatment or diagnosis of a disease or condition that affects or is manifested in not more than 8,000 individuals in the United States per year as eligible for Humanitarian Device Exemption.
Are you looking for regulatory support for your drug in rare diseases? Talk to us today. Provide details of your requirements and we will connect with you.
References
- https://www.fda.gov/industry/developing-products-rare-diseases-conditions/designating-orphan-product-drugs-and-biological-products
- https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/clinical-superiority-findings
- https://www.fda.gov/industry/designating-orphan-product-drugs-and-biological-products/frequently-asked-questions-faq-about-designating-orphan-product
- https://info.evaluate.com/rs/607-YGS-364/images/EvaluatePharma%20Orphan%20Drug%20Report%202019.pdf?mkt_tok=eyJpIjoiWWpVMk1UVmtNRFpqT0dFeiIsInQiOiIrcmZ3QjNwamZWWVwvZ1ZkcU5XS2E3Rk5oNXA5MXZJVUVCRitMQXpQd0sxMGJPU0JhdGRWbVJQQkZrc0xZNDNPSXRNM09wMGh2OEFXNXFNN1wvb1plT
- https://www.raps.org/news-and-articles/news-articles/2020/1/fda-2019-continues-uptick-in-orphan-drug-approval