What is the best EDC system / eCRF you’ve ever used? Review of online user-initiated surveys on social media

A case record form (CRF) is a great and necessary tool used in clinical trials to collect data from each participating patient. CRF (also referred to as case report form) has historically been on paper (unlike electronic ones these days). Traditionally, something like carbon copy paper, where the sponsor can utilize one sheet for the Trial Master File (TMF). In some geographies, smaller studies still use paper CRFs. But, mostly, there is a push and inclination to use electronic CRFs(eCRF) or Electronic Data Capture (EDC) in large/global studies.

What are the advantages of electronic data capture (EDC) or eCRF?
Some are listed below. Do you want to add more?
- Easy availability of data – Trial teams, monitors, and auditors can review the data wherever and whenever they want to.
- Inbuilt plausibility to ensure the appropriateness of data – To ensure proper data entry, the sponsor can make predefined checks.
- Faster accumulation and data transfer – Reduces data entry time, particularly from external devices.
- Easy sharing and exchange of information – Users can share data quickly on capture, process, and acceptability with relevant personnel.
- Real-Time Automated Edit Checks – Has a clear advantage over paper CRFs.
- Facilitate Analysis – Data collected in eCRF are linked with one form to another form for analysis.
- Rapid formal reporting – Authorized personnel can access, download, and print status reports any time throughout the study and on completion.
- Unlimited security possibility – Any level of security in storage and built an access system.
- Higher data quality – increased statistical power.
- PRO readiness – Ability to work seamlessly with PRO.
An excellent presentation from Liora Bosch of Omrix Biopharmaceuticals, a J&J company, provides a great case for eCRF utility, key considerations, and challenges.
What are common EDC systems available in the market?
There are different kinds of EDC systems developed by various companies, and more are still coming up.
Somewhere in the western world, a clinical trial team of an organization was looking to buy an electronic data capture (EDC) system for their clinical trial projects. There were many choices in the market, and efforts were on to seek feedback on previous experien6ces with these products. One of the team members got an interesting thought to seek this feedback quickly and much more reliably. Why not ask other clinical researchers on a social media group? This generated a pool of responses containing unbiased feedback of user experience with currently marketed EDC systems.
Here are some of the known EDC systems in the market.
- Rave from Medidata
- Inform from Oracle
- Oracle RDC from Oracle
- nowEDC from Datatrial
- Datalabs EDC from Perceptive(Subsidiary of Parexel)
- Macro from Elsevier
- QDS EDC from QDS
- Data Trak EDC from Data Trak
- TrialMaster from Omnicomm
- Encapsia from Cmed
- IBM EDC from IBM
- Target Health EDC from Target Health
- Veeva Vault EDC from Veeva
- eCaseLink from DSG
- Medrio EDC from Medrio
- Captivate EDC from clinCapture
- REDCap EDC from REDCap Cloud
- Nukleus EDC from TCD eClinical
- Bioclinica EDC from Bioclinica
- Castor EDC from Castor
- iMedNet EDC from MedNet
While most of these EDC systems bring advantages suggested earlier, their acceptability in the market differs significantly.
Are you looking for the best EDC/eCRF system?
Fill out the form below to connect with us
User experience – a key factor in the acceptability of eCRF systems
User experience – the overall experience of a person using a product e.g. EDC or eCRF, especially in terms of how easy or pleasing it is to use, drives a user to like or dislike that product.
It generates perceptions of system aspects such as
- Utility,
- Ease of use and
- Efficiency
for that particular user.
- It means that the acceptability of a product, including EDC systems, can be a total of various aspects of human-computer interaction and product ownership.
- For a product like eCRF / EDC, user experience becomes more crucial, with timely submission, review, and stringent data acceptability requirements in clinical research.
- An EDC system needs to be taken up, understood, and utilized fully by the user. The learning curve should be acceptable and not too steep.
Learn more about various software and data management tools used in clinical trials
Clinical researchers are aware and the word spreads fast
Considering the complexities of clinical trials, most of the users in the industry undergo fast realization of how user-friendly a system is. With a need to use these systems quite frequently by different kinds of users – clinical study coordinators, investigators, clinical trial monitors, etc. to name a few, any product is likely to generate an impression rather quickly.
What becomes more interesting is how this impression spreads across the industry.
Any prospective buyer (clinical research organization or a trial sponsor) is likely to take feedback from various resources before opting for their best choice. Some of these resources could be
- Experience of employees
- Feedback from friends, ex-colleagues, and associates of employees
- Online reviews of products
- Live demonstration of products
- Sponsor requirements
This survey makes sure that a good product always gains favorable words and applause quicker than a not-so-good product getting an unfavorable one. Thus, tapping the impressions of users, as they come, is of great importance.
Click here to explore ways to accelerate your clinical development
Story of a user-initiated survey on EDC
Somewhere in the western world, a clinical trial team of an organization was looking to buy an electronic data capture (EDC) system for their clinical trial projects. There were many choices in the market and efforts were on to seek feedback on previous experiences with these products.
One of the team members got an interesting thought to seek this feedback quickly and much more reliably.
Why not ask other clinical researchers on a social media group?
LinkedIn, Facebook, and other social media have become virtual community centers for people. They are not only able to stay in touch with their friends, colleagues, and known persons but can connect with various other (unknown) people with similar interests by joining a social media group.
Within clinical research too, there are various groups formed to share information, knowledge, and, very often, a smile.
On a fine evening, one such group received a question from a group member – “What is the best EDC system you’ve ever used?”.
This question generated a flood of responses from many group members. Many people shared what they thought was the best EDC system. A lot of them shared their experiences with such products with more elaborated comments.
The Series of responses received for this survey generated pooled unbiased feedback on various marketed EDC platforms and eCRF systems. Considering the importance of early understanding of user experience, this feedback could be very interesting for clinical researchers, as well as developers of such EDC systems.
Results of the survey
They analyzed the survey responses. They considered all the product comments. Responses/comments/feedback on these products were also listed and classified into three categories.
- Select’: They considered the positive feedback from the products.
- ‘Reject’: A list of negative responses was listed.
- ‘Detest’: In this category, they considered the strong dislike than just rejection from the users.
This survey received a total of 207 responses at the time of preparation of this article. Here is the result of the analysis performed using the above-referred categories.
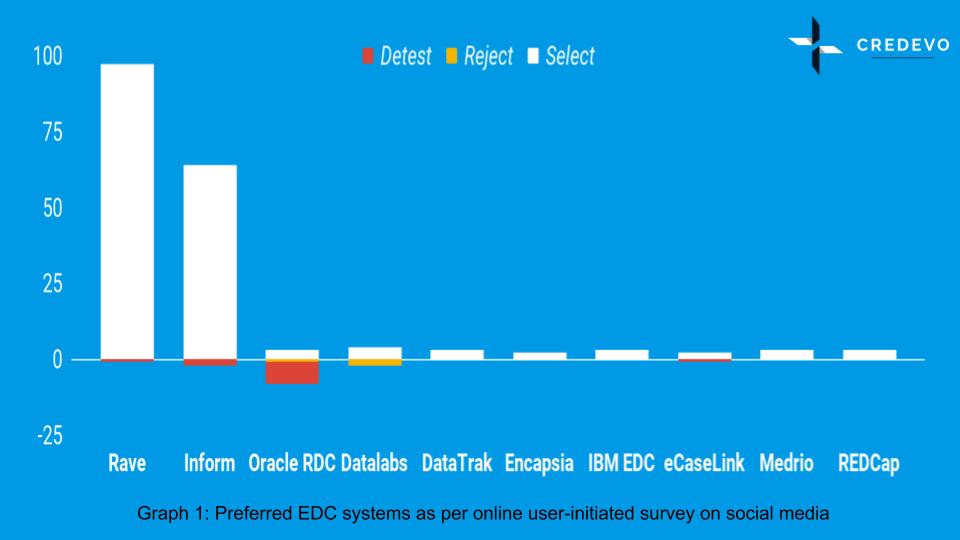
- The majority of respondents prefer Rave from Medidata and Inform. Roughly half of the responses indicated a favorable view of Medidata, while more than 30% of feedbacks favored Inform.
- There were some negative responses as well. But, it’s interesting to note that Rave and Inform had negligible negative feedback.
- Oracle RDC was the only product with more negative responses than positive ones.
Results of another survey on EDC systems other than Rave
A different user performed a survey to find recommendations on products other than a rave. Since Rave stood out as an undisputed winner among the respondents in the earlier survey, the findings of the second survey become crucial to assess the second choice for an eCRF system.
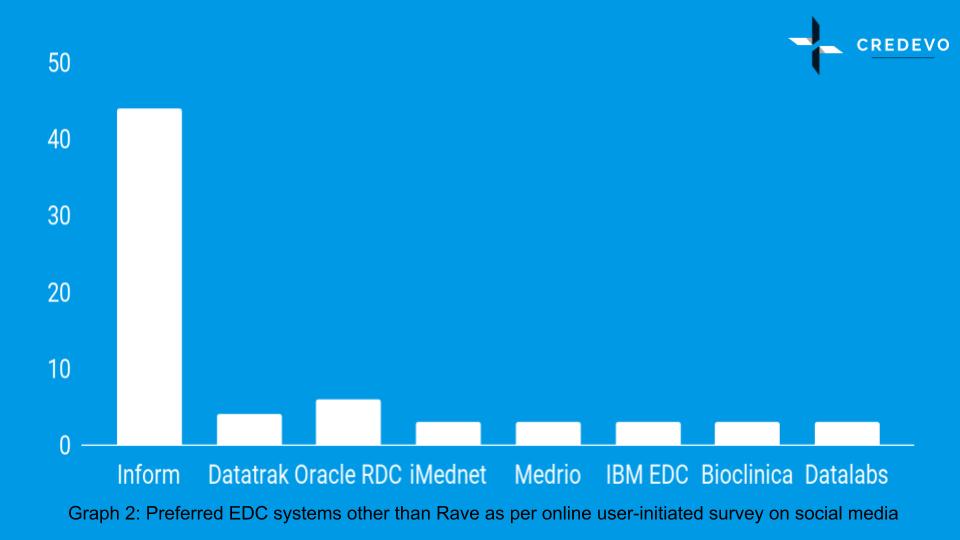
Among 93 responses, there were 44 positive responses for Inform, which is nearly 50%. Inform again stood out as the clear choice of the EDC platform.
However, it is also interesting to note that the second choice of users after Inform, is Oracle RDC here.
What type of support are you looking for in your clinical trials?
Talk to us today. Provide preliminary details below, and we will help you achieve data management objectives for clinical trials.
References
- What is an eCRF in clinical trials? – Illingworth Research Group
- Case report form – Wikipedia
- Inform Electronic Data Capture (EDC) and Data Management System
- Key Considerations and Challenges of EDC in the Implementation and Statistics of Clinical Trials – Presentation by Liora Bosch
- Comparison between paper CRF and eCRF | ClinZen Clinical Trial Blog
- Mobile electronic versus paper case report forms in clinical trials: a randomized controlled trial