Potential and Challenges With Clinical Trial Sites In Asia & West

Trial managers worldwide have been focusing on specific regions for placing their clinical trials. So much so that about 60-70% of clinical trials globally are conducted in the USA and Europe alone. However, this is slated to change or should change, if one consults a recent white paper published by Frost & Sullivan.

The white paper “ASIA: PREFERRED DESTINATION FOR CLINICAL TRIALS” has some interesting observations to make. It highlights research on why Asia is a key destination for clinical trials. The observations made include factors pertaining to quality, cost, and timelines – the three most important considerations for any project. It also identified important challenges and issues, with conducting research in Asia.
We compare these observations for Asian and Western sites below.
Quality – trained investigators, infrastructure, and sites
Site infrastructure is always a key factor for clinical trials. Here are some interesting facts about it.
- Western centers are traditionally considered to have an edge over Asian sites in terms of quality standards. The hospital infrastructure and systems at western sites are well established and have been in existent for a long.
- Asia is picking up fast too, particularly in infrastructure. Asia now has numerous specialized clinical trial centers with advanced equipment and technology.
- Fast-growing Asian economies are engaged heavily in enhancing legacy information technology (IT) systems and are at the forefront of innovation.
- However, issues might exist in smaller Asian cities or fast-growing cities with insufficient infrastructure investment. Technology dependent data capturing methods and backup infrastructure may need to be planned.
- Another factor to consider is the logistics complexities in Asian megacities. Traffic congestion is an important factor, while planning road transport logistics to ensure diagnostic samples or investigational products are not compromised. CRO vendors need to have different approaches for time-sensitive transportation, such as multiple drug depots or temperature-controlled couriers. This will help ensure crucial patient samples or drugs are not affected in transit.
Another important factor for consideration is data acceptability. Frost and Sullivan’s white paper also identified the potential, quality standards, and capabilities of Investigators and sites in Asia.
- Western sites have more experience and established acceptability for US FDA and EMA regulatory submissions.
- Data from clinical trials in Asia is now routinely accepted as well for US FDA and EMA regulatory submissions.
- It was reported in the white paper that the data from inspections conducted in Asia by US FDA and EMA show low levels of adverse findings versus the US or European Union (EU).
- Many Asian sites are global leaders in relevant therapeutic areas. Some examples include National Taiwan University (NTU) Hospital (cardiovascular), NTU’s College of Medicine (Oncology), and many other sites in Japan, China, Singapore, and South Korea for stem cell therapy and regenerative medicine research.
Timelines – faster recruitment and retention
The clinical trials process can be time-consuming. The average time from clinical testing to gaining approval to market the drug is roughly 7 to 8 years, while the full process from discovery to registration takes between 11 and 15 years.
Failure to meet patient numbers or timelines can delay product launches. A 2013 analysis found that almost 57% of trials fail due to low patient accrual rates that could translate into huge financial losses.
White paper identified changing trends and views for timelines at sites in these regions.
- Low participation has encouraged more biopharmaceutical companies to pursue clinical trials beyond the US and Western Europe.
- A number of examples now exist to support the faster recruitment potential of Asian sites. Like, one trial for HIV-associated cryptococcal meningitis, where Thailand trial sites were added as rescue sites. This added an average of 4 patients per site in Thailand over 3 months compared with 1 per site in the United States.
- The lengthy review times of both the US FDA and the EMA add to the drug development process.
- With large treatment-naive patient pools, comparable incidence, and prevalence of Western diseases, Asian sites are able to add more patients to the trials now.
- Low healthcare spends by many governments in Asia makes clinical trials an attractive way for patients to access innovative therapies in these countries.
Regulatory approvals – Opportunities and challenges are high in Asia too
While, lengthy review times of western agencies have been recognized as a factor that affects timelines in respective regions, in Asia it’s the heterogeneous nature of regulatory regimes that increases complexities.
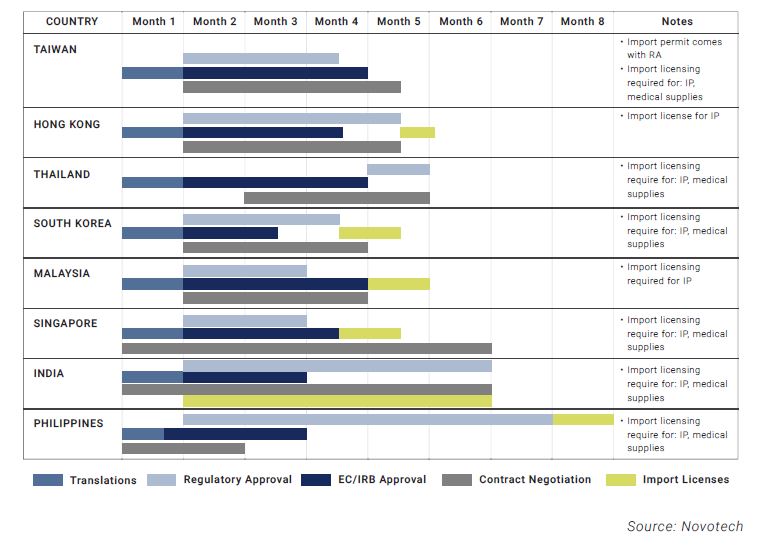
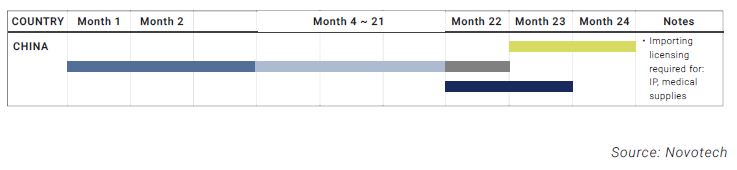
- The Institutional Review Board (IRB) approval, regulatory, import licensing, and contract negotiations are undertaken simultaneously in some countries, while others carry them out separately.
- Each country in Asia (which are smaller than US or EU regions) have their own regulations and require individual considerations. This may add more complexities.
- Asian countries also have varying requirements for local language translation, import or export licensing, and data on local patients.
- As a positive point, many countries in Asia have regulatory approval time frames that are competitive with Western counties, and more streamlined to ensure timely and predictable regulatory approvals, as shown above.
Cultures issues – a barrier to watch in Asia
There are various cultural factors, many unique to Asia, that are required to be considered for clinical trials.
- Local languages and English variability: For global trials, most Asian sites may require translation of trial documentation, although in some counties this may only apply to patient materials. To ensure that the meaning of critical documents is preserved, one may want to invest in back-translation from the local language to English as well.
- Cultural norms: Professional position, gender, and age play a major role in many Asian cultures. Maintaining a good reputation at the workplace and creating an individual and community view is also important.
- Saving face: This is pivotal in business negotiations, and in developing and maintaining relationships. For example, it is helpful to have senior staff members communicate non-compliance to principal investigators, and junior employees to submit improvement ideas via process-based templates.
- Multiple ethnic groups, religions, and belief systems: Vendors should have a good understanding of different cultural beliefs during the planning of clinical trials because these could affect patient recruitment and retention. For example, Chinese New Year and Ramadan festivals in Southeast Asia are widely celebrated and could impact trial schedules.
- High trust in physicians: Patients in Asia respect doctors and rely on their guidance in determining medical treatments. Physicians play a vital role in recruiting patients in these nations, and this high level of trust may contribute to low levels of patient attrition.
- Perceptual reporting: Many Asian cultures have differences in the way they report pain, moods, and emotions e.g. depression, mania, and anxiety. Validated cultural equivalence surveys may therefore be important in the design and analysis of certain trials.
With Credevo, it’s very easy to connect with Asian, US, Canadian, Australian, and New Zealand sites. Within Asia, you can reach investigators in China, Korea, Taiwan, Malaysia, Singapore, Thailand, Vietnam, Philippines, India, Srilanka, Bangladesh besides other countries in the region. For more details contact us at [email protected] or fill the details in the form below
Cost – an interesting advantage of Asian sites
Asia offers lower costs for clinical trials.
For example,
- Costs in China and India are 25% to 40% lower than that of Western countries.
- The cost of trials in the EU has grown significantly due to higher insurance fees and increased administrative and resource costs for the trials.
- The doctor visits, medical treatments, and procedures tend to cost less in Asian countries, as shown below.
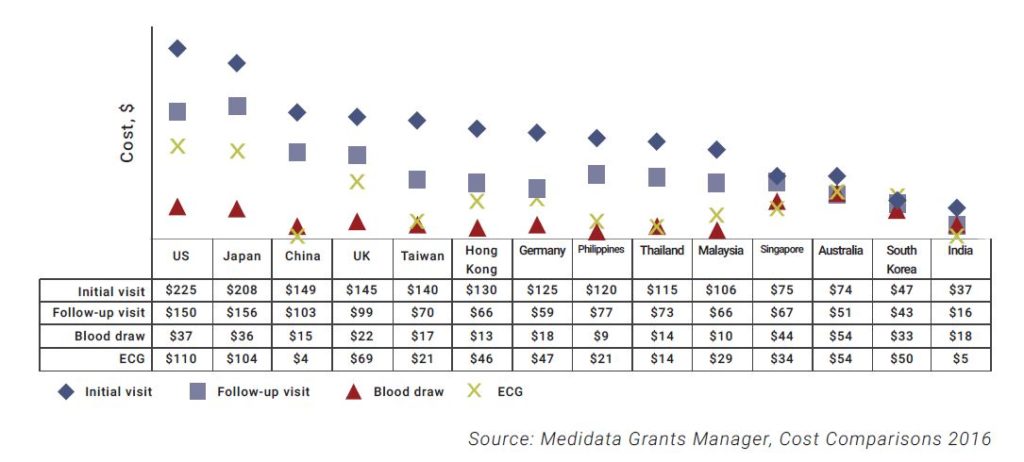
To quote as an example, an ECG procedure in the US with interpretation and report costs around $110 on average, while the same report would only cost $4 in China.
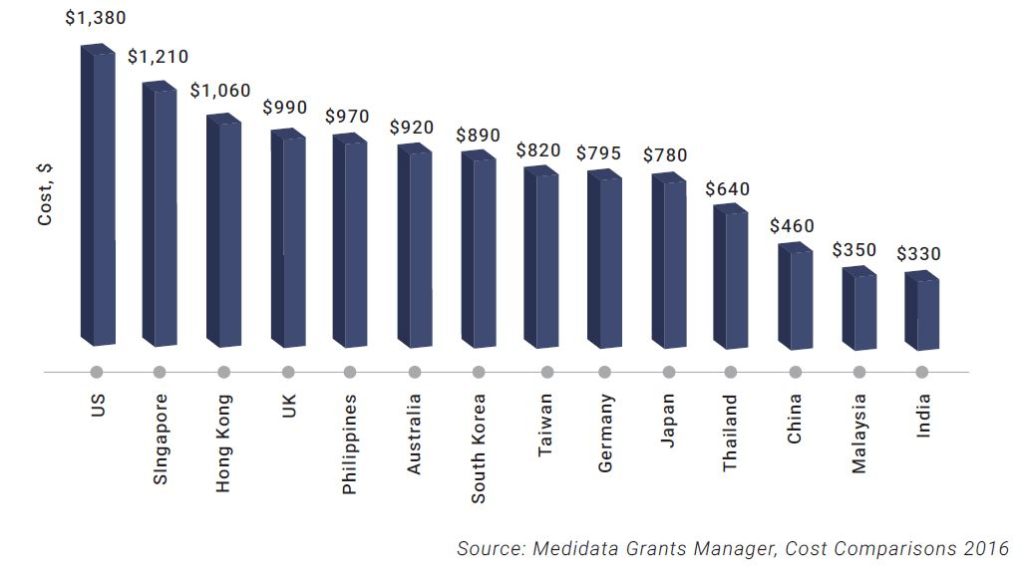
On the other side, there is a cost factor, where western sites do better than Asian ones.
In most Asian countries sponsors are required to pay for the standard of care. However, in the US, Europe, and Australia, the standard of care is generally provided by the patient’s normal payer during a clinical trial.
Nevertheless, the total cost of running studies in Asia continues to provide cost savings in the region of 30% to 40% as compared with the US and Western Europe.
Would you like to connect with sites for your clinical trials?
Credevo has extensive experience and expertise in performing such selection for our clients through feasibility studies. Credevo often works as an extended arm of pharma / biotech companies in building clinical development strategies and selecting the right paths for successful and accelerated completion of clinical trials.
Provide your query details and email to connect with us and explore our support for your clinical trials.
3 thoughts on “Potential and Challenges With Clinical Trial Sites In Asia & West”
Comments are closed.