Clinical Trial Feasibility: Breast Cancer Studies

Worldwide, breast cancer is the most common invasive cancer in women. Along with lung cancer, breast cancer is the most commonly diagnosed cancer, with 2.09 million cases each in 2018, and Breast cancer affects 1 in 7 (14%) of women worldwide.

What is breast cancer?
Breast cancer is a cancer that develops from breast tissue. The breast cancer most commonly develops in cells from the lining of milk ducts and the lobules that supply these ducts with milk.
Cancers developing from the ducts are known as ductal carcinomas, while those developing from lobules are known as lobular carcinomas and there are more than 18 other subtypes of breast cancer. Some, such as ductal carcinoma in situ, develop from pre-invasive lesions.
What are the signs and symptoms of breast cancer?
Signs of breast cancer may include
- a lump in the breast,
- a change in breast shape,
- dimpling of the skin,
- fluid coming from the nipple,
- a newly-inverted nipple, or
- a red or scaly patch of skin.
and in the patients with distant spread of the disease,
- there may be bone pain,
- swollen lymph nodes,
- shortness of breath, or
- yellow skin
What are the risk factors for developing breast cancer?
Risk factors for developing breast cancer include
- being female,
- obesity,
- a lack of physical exercise,
- alcoholism,
- hormone replacement therapy (HRT) during menopause,
- ionizing radiation,
- early age at first menstruation,
- having children late in life or not at all, older age,
- having a prior history of breast cancer, and
- a family history of breast cancer.
Prevalence of breast cancer
- Breast cancer is the most common cancer in women both in developed and developing countries.
- It was estimated that over 508 000 women died in 2011 worldwide due to breast cancer (Global Health Estimates, WHO 2013).
- Although breast cancer is thought to be a disease of the developed world, almost 50% of breast cancer cases and 58% of deaths occur in less developed countries
- The breast cancer survival rates vary greatly worldwide, ranging from 80% or over in North America, Sweden, and Japan to around 60% in middle-income countries and below 40% in low-income countries.
Clinical trials on breast cancer worldwide
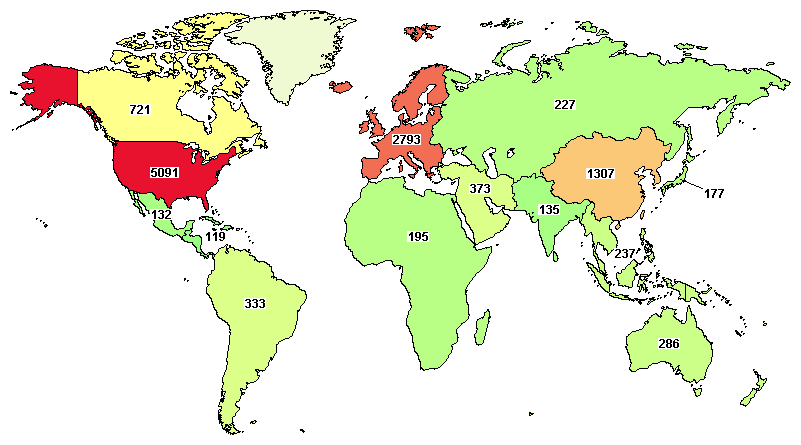
A total of 10,214 clinical trials were registered around the globe, where major trials are going in the United States (5,091), Germany (518), Canada (721), France (912), and Spain (638).
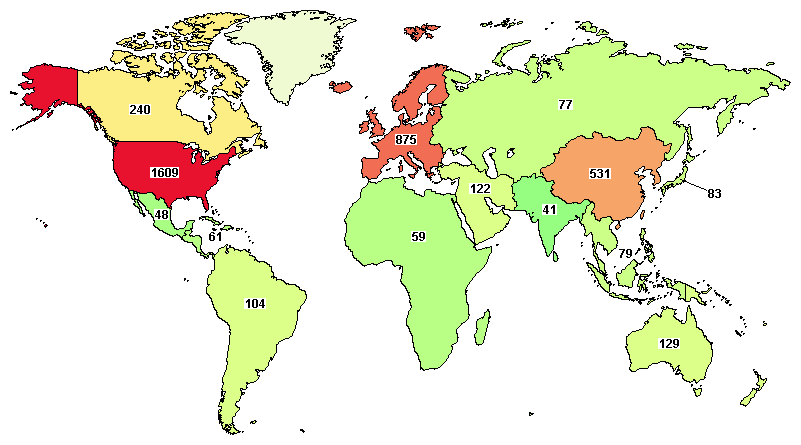
The total ongoing trials on AML are 785, where major clinical studies are in the United States (1,609), France (311), China (320), Spain (226), and Canada (240).
Feasibility Results
Presenting a tentative recruitment rate obtained from clinical trial feasibility performed on Credevo.com for two clinical trials in breast cancer. The rate was found to be varying between 2-9 across the Asia-Pacific region. Take a look at the below figures (details are being shared with approval from report owners).
Metabolic Breast Cancer Phase III Clinical Trial
| Country | Average Recruitment Rate Per Site Per Month |
| Philippines | 4 |
| Thailand | > 4 |
| India | 2 |
| Russia | 2 |
| Canada | 5 |
Breast Cancer Phase III Clinical Trial
| Country | Average Recruitment Rate Per Site Per Month |
| Philippines | > 6 |
| Vietnam | 9 |
| India | > 5 |
| Russia | > 4 |
| South Korea | 3 |
Why this matters
- Today, breast cancer is one of the most studied and researched areas for the clinical development of drugs.
- Researchers are trying their best to find clinical sites and investigators, where they can conduct clinical trials quickly and with reliable data. However, most of the options available are from existing sites that are limited and saturated. There is a definite need to find more clinical sites.
- Feasibility at Credevo can help trial managers get quick and easy foresight into what they can expect if they choose particular regions for their clinical trial in breast cancer patients.
How big is this research area
- Today, breast cancer is one of the leading causes of mortality in females worldwide.
- In the United States, it’s been reported to be the fourth biggest cause of deaths in females (https://seer.cancer.gov/statfacts/html/breast.html). Unsurprisingly, globally, there are many research programs working to create successful treatments for breast cancer.
- Many pharmaceutical companies in the US, Europe, and other regions are engaging in the development of drugs, innovative as well as generic, for the treatment of this deadly condition.
Researchers’ Challenge
To complicate the clinical development of drugs for breast cancer, it has become difficult to find competent research centers that can recruit patients faster and complete trials without much delay.
Researchers are approaching various local, regional, and international centers to ensure that clinical trials get completed quickly.
Regions for Research
Besides the United States and Europe, Asia-Pacific, India, China, and Russia have come up as preferred regions for conducting these trials. However, the uncertainty of recruitment rate and difficulty in finding sites in such regions is one of the main challenges for trial managers worldwide.
Contact Investigators From This Feasibility Assessment
Would you like to contact these investigators and assess feasibility with them for your clinical trial? or Do you need any support in conducting such clinical trials?
Just provide your email below and connect with us. Don’t worry, your email will not be published or shared.