Exploring The Asia-Pacific Generic Drug Landscape: Key Insights

The Asia-Pacific region, with its growing population, growing healthcare needs, and attempts to cut costs, is one of the major players in the generic drug market. In recent years, the Asia-Pacific region has seen a significant push towards using generic medications to cut costs and improve efficiency in healthcare systems.

This trend is evident across various countries in the Asia-Pacific region, where governments have set clear targets for increasing the use of generic drugs. As a result, generic prescriptions now make up over 70% of total prescriptions in many Asia-Pacific countries.
The robust pharmaceutical industry in Asia-Pacific countries contributes significantly to the region’s prominence in generic drug manufacturing. These countries have advanced manufacturing, skilled labor, and established supply chains, ideal for large-scale, cost-competitive generic drug production.
In this comprehensive overview, we’ll explore insights and essential information you need to know about the growing landscape of generic drugs in the Asia-Pacific region.
The market for generic drugs in the Asia-Pacific region
The Asia-Pacific generic drugs market is predicted to reach USD 132.4 billion by 2029, up from USD 88.76 billion in 2024, with a CAGR of 8.34% from 2024 to 2029.
Governments are actively working to procure medications at more affordable prices. For instance, Japan has seen a substantial increase in generic drug usage, with plans to raise it from 68% to 80%. This shift towards generics reflects a broader strategy of achieving more with less healthcare spending across the region.
The following charts give a clearer understanding of the rise of generic drugs in the Asia-Pacific region.
Global generic drug sales from 2021–2024
From 2021 to 2024, the worldwide sales of generic drugs experienced significant growth. In 2024, Asia-Pacific countries continued to lead in sales, reflecting a growing trend toward affordable medication options. This surge underscores the increasing importance of generic drugs in providing accessible healthcare solutions globally.
Global generic drug sales from 2021–2024
Generic drug market in Asia-Pacific countries in 2023
As of 2023, the Asia-Pacific region’s generic drug market is thriving and represents a turning point in the healthcare system in the area. China and India are leading the way in this market, which is expanding rapidly due to their extensive pharmaceutical industries in the generic drug market.
Asia-Pacific will remain the top growth region for generic drugs in 2024. With consistent expansion, the region exemplifies a dynamic market for affordable pharmaceuticals, catering to diverse healthcare needs.
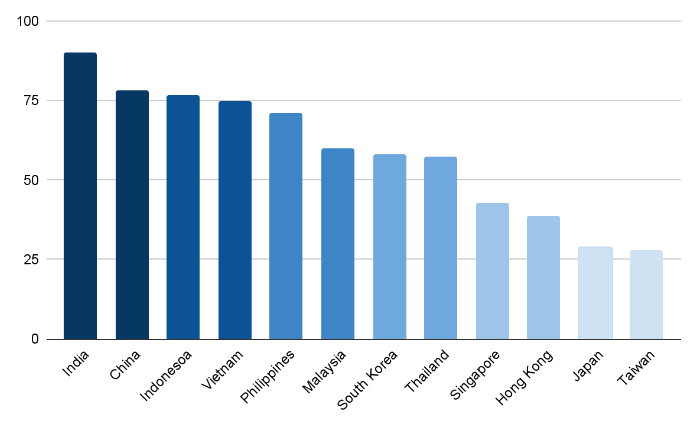
Generic drug market in Asia-Pacific countries in 2023
Growth of generic drugs in the Asia-Pacific region from 2014 to 2025
The Asia-Pacific region is experiencing rapid growth in the generic drug market, with a steady increase in Compound Annual Growth Rate (CAGR) values from 2014 to 2025. According to recent data, this region’s CAGR for generic drugs has been remarkable, indicating a robust industry expansion. By 2025, the market for generic drugs is expected to grow to $500 billion.
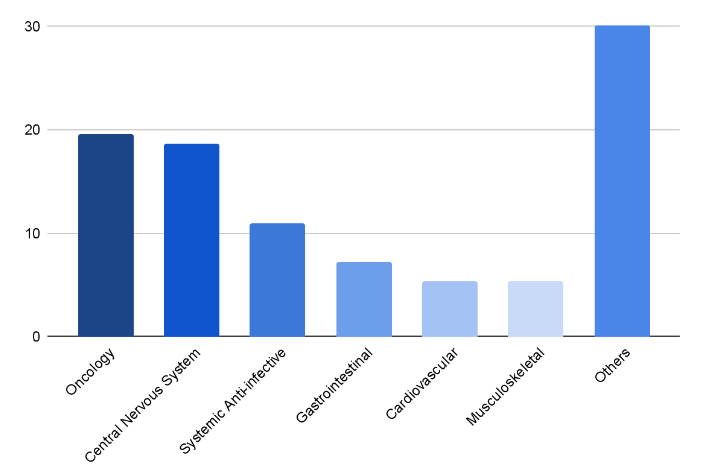
Growth of generic drugs in the Asia-Pacific region by therapeutic areas from 2020 to 2026
The rise in generic drug sales in the Asia-Pacific region from 2020 to 2026 across several therapeutic areas is necessary to understand. Across all therapeutic domains, generic medications are becoming popular because of their many advantages, such as affordability and availability.
Among these areas, central nervous system disorders stand out as the most prominent in terms of generic drug usage. This trend underscores the broad adoption of generic medications as a viable treatment option, highlighting their importance in providing affordable healthcare solutions across the Asia-Pacific region.
Growth of generic drugs in the Asia-Pacific region by therapeutic areas from 2020 to 2026
Regulatory background of generic drugs in Asia-Pacific countries
The regulatory environment of generic drugs in Asia-Pacific countries presents unique challenges and opportunities. The following table highlights the primary regulatory authorities and provides a summary of the approval processes involved. Understanding these regulations is crucial to ensure that generic medications are accessible, safe, and effective in meeting diverse healthcare needs in the Asia-Pacific region.
| Country | Regulatory body | Basic requirements to register a generic drug |
|---|---|---|
| Japan | Pharmaceuticals and Medical Devices Agency (PMDA) | Need to submit comprehensive data on quality, safety, and efficacy, including bioequivalence studies. |
| South Korea | Ministry of Food and Drug Safety (MFDS) | Need to submit data on quality, safety, and efficacy, along with bioequivalence studies. |
| Australia | Therapeutic Goods Administration (TGA) | Need to submit data demonstrating the quality, safety, and efficacy, including bioequivalence studies. |
| India | Central Drugs Standard Control Organization (CDSCO) | Need to submit data on quality, safety, and efficacy, along with bioequivalence studies. India also accepts some bioavailability studies instead of bioequivalence studies for some products. |
| Thailand | Food and Drug Administration (Thai FDA) | Need to submit data on quality, safety, and efficacy, including bioequivalence studies. |
| Singapore | Health Sciences Authority (HSA) | Similar to other countries, submission of data on quality, safety, and efficacy, along with bioequivalence studies. |
| China | National Medical Products Administration (NMPA) | Need to submit data on quality, safety, and efficacy, including bioequivalence studies. China also requires local clinical trials for generic drug registration. |
Please click the link below for more details regarding the generic drug registration process in Asia-Pacific countries.
Factors driving the growth of generic drugs in the Asian Pacific region
The growth of generic drugs in the Asia-Pacific region is influenced by various factors, reflecting a shift towards affordable and accessible healthcare. Understanding these drivers is crucial to understanding the pharmaceutical landscape in the Asia-Pacific region.
The following are some factors that drive the growth of generic drugs in this region.
- Affordability: The affordability of generic drugs is a primary driver of their growth in the Asia-Pacific region, offering cost-effective alternatives to brand-name medications.
- Government initiatives: play a pivotal role in driving the growth of generic drugs in the Asia-Pacific region, with policies promoting the use and manufacturing of generic drugs.
- Increasing disease burden: The increasing disease burden in the Asia-Pacific region underscores the importance of generic drugs in addressing public health challenges and providing essential treatments for prevalent diseases.
- Pharmaceutical industry: The pharmaceutical industry’s involvement and investment in generic drug manufacturing and distribution contribute significantly to its growth in Asia-Pacific countries.
Challenges in the generic drug market in the Asia-Pacific region
Despite growth, challenges in the Asia-Pacific generic drug market affect medication accessibility, affordability, and quality. Understanding these challenges is crucial to address gaps and improve the outcomes.
- Quality concerns: Quality concerns pose significant challenges in the Asia-Pacific generic drug market, impacting patient safety and confidence. Addressing these concerns requires stringent quality control measures and effective regulatory oversights.
- Regulatory hurdles: Complex regulatory frameworks present significant hurdles for generic drug manufacturers in the region, delaying market entry and hindering accessibility. Streamlining regulatory processes are essential to overcome these barriers.
- Intellectual property rights (IPR) issues: Patent disputes and infringement concerns, create legal and market barriers for generic drug manufacturers in the Asia-Pacific region. Balancing innovation incentives with the need for affordable healthcare requires robust legal frameworks and mechanisms to resolve IPR disputes effectively.
- Price erosion and profit margins: Price erosion and shrinking profit margins challenge the sustainability of generic drug businesses in the Asia-Pacific region, affecting investment in research and development. Implementing pricing policies that balance affordability with industry viability is essential to ensure long-term availability.
The future of generic drugs in Asia-Pacific countries
The future of generic drugs in the Asia-Pacific region holds promise amidst evolving healthcare landscapes. Several factors shape this trajectory, indicating opportunities for growth, innovation, and enhanced accessibility of medications. Understanding these dynamics is vital for the future of generic drugs in APAC.
- Technological advancements and innovations: Technological advancements and innovations are poised to revolutionize the generic drug market in the Asia-Pacific region, driving efficiencies in manufacturing, formulation, and delivery methods.
- Market expansion: Market expansion efforts are crucial for the future of generic drugs in the Asia-Pacific region, facilitating broader accessibility and uptake of affordable medications.
- Collaborative efforts: Governments, pharmaceutical companies, and healthcare providers are essential for advancing the future of generic drugs in the Asia-Pacific region.
- Patient education: Patient education initiatives are critical for the future of generic drugs in Asia-Pacific countries, empowering individuals to make informed healthcare decisions.
Conclusion
In conclusion, understanding the dynamics of the generic drug market in the Asia-Pacific region is crucial for the healthcare spectrum. From technological advancements to collaborative efforts and patient education, various factors shape the landscape of generic medications. Addressing challenges and embracing opportunities can improve healthcare accessibility, affordability, and quality, benefiting patients and the industry.
Do You Want To Know More About The Generic Drug Market In The Asia-Pacific Region?
Are you considering expanding your generic drug presence into the Asia-Pacific region and have questions? Credevo offers comprehensive services for drug development and regulatory approval in this region, providing expertise from initial development to navigating regulatory hurdles, streamlining the process. Provide your requirements in the form below to connect and talk with our expert team.