Guide To Medical Device Approval Process In Taiwan: Regulations, Requirements & Steps

For entry into the Taiwan market, all medical devices require pre-market approval. The medical device market is growing in Taiwan, but getting into the nation requires strict adherence to regulations set forth by the Taiwan Food and Drug Administration (TFDA). Manufacturers must register their products with the Taiwan Food and Drug Administration (TFDA) to sell medical or in vitro diagnostic (IVD) devices in Taiwan.

The size of the medical device market was estimated at USD 71.1 billion in 2023, with a compound annual growth rate (CAGR) of 10.7% from 2023 to 2030. By 2030, the market is expected to reach USD 160.34 billion.
First, let us walk you through Taiwan’s regulatory system and the key elements of the country’s medical device regulatory requirements.
Regulatory authority for the registration of medical devices in Taiwan
- The Taiwan Food and Drug Administration (TFDA), a division of the Ministry of Health and Welfare (MOHW), regulates medical devices under the Pharmaceutical Affairs Act.
- In addition to reviewing Quality System Documentation (QSD) supplied by foreign manufacturers, MOHW conducts on-site inspections for local manufacturers. The TFDA has to provide a registration certificate for any imported medical device.
- As of May 2020, the regulatory system will be governed by the new Medical Devices Act.
What is the definition of the term ‘medical device’?
- According to the Medical Devices Act, the term “medical devices” refers to instruments, machines, apparatus, materials, software, reagents for in vitro use, and related articles thereof, whose design and use achieve one of the following primary intended actions in or on the human body by other than pharmacological, immunological, metabolic, or chemical means:
- Diagnosis, treatment, alleviation, or direct prevention of human diseases.
- Modification or improvement of the structure and function of the human body.
- Control of conception
Is it necessary to have a local authorized representative in Taiwan to register a medical device?
Yes, foreign medical device and IVD manufacturers without a physical office in Taiwan must have a local authorized representative (LAR) in Taiwan to register medical devices. The LAR must be a legal entity in Taiwan that holds a Business License and a Medical Device Business Permit.
The LAR holds various responsibilities, including:
- Assist in Quality System Documentation (QSD) Certification.
- Responsible for filing Import Authorization requests to the TFDA for the distributor.
- Submitting the registration application to the TFDA.
- Replying to TFDA inquiries.
- Giving TFDA updates on the device.
- Ensuring that the device complies with TFDA regulations.
- Addressing any post-market issues.
Need regulatory support in the Asia-Pacific regions? Click here to connect with our team and discuss your regulatory needs.
Medical device classification in Taiwan
Manufacturers must classify their products before applying for registration.
- Taiwan uses the International Medical Device Classification System (IMDRF) to categorize medical devices based on risk, intended use, duration of contact with the human body, and invasiveness.
- Medical devices are classified as non-in-vitro diagnostic devices and in-vitro diagnostic devices (IVDs).
- Additionally, they may be classified as Class-I or Class-II/III based on their risk level.
| Medical Device Class | Risk | Examples | Type of review |
|---|---|---|---|
| Class I | Low risk | Thermometers, Forceps, and Surgical Instruments | Administrative |
| Class II | Moderate risk | Endoscopes, ECG Machines, and Dental Instruments | Administrative and Technical |
| Class III | High risk | Implantable Pacemakers, Defibrillators, Artificial Heart Valves | Administrative and Technical |
| Class III New (No predicate device previously approved by TFDA) | Administrative and Technical |
- Identifying a predicate device, which can be accomplished by browsing the public database or working with a local partner to discover similar approved devices, is a crucial step in the Taiwan registration process.
- A registration application for Class-I devices must be filed to the TFDA for approval. Class-II and III devices also require the submission of a dossier containing technical information, product test reports, and any necessary clinical data.
IVD device classification in Taiwan
| IVD Class | Risk | Type of review |
|---|---|---|
| Class I | Low risk | No Registration required |
| Class II | Moderate risk | No Registration required |
| Class III | High risk | Administrative and Technical |
| Class III New (No predicate device previously approved by TFDA) | Administrative and Technical |
QSD requirements for medical & IVD devices
- In addition to medical device registration in Taiwan, the manufacturing site must register for Quality System Documentation (QSD) ISO 13485.
- In Taiwan, a QSD license (issued upon QSD registration approval) is analogous to Good Manufacturing Practice (GMP) for Medical Devices.
- QSD is not necessary for Class-I (non-sterile) medical devices.
Common documents required for QSD application
The following are the documents required for QSD registration approval:
- Quality System Documentation (QSD) ISO 13485 certificate
- Notified Body letter – original hardcopy required
- QSD Statement (TFDA Template – basic information on the manufacturer) – requires original hardcopy.
- Manufacturing Process Flow Chart
- MDF or DMR (Including the information about the indications for use, specification, manufacturing, packaging, storage, transport, distribution instruction, quality control instruction, installation instruction, and service instruction).
Documents necessary for registration
The following are the documents required for registration:
- A copy of the GMP/QSD Compliance Letter.
- Application form
- Copies of Chinese labeling, instructions for use, and packaging inserts
- Copies of product structure, material, specifications, intended use, and drawings
- A copy of the Pharmaceutical License for Medical Device Manufacturer/ Distributor/ Agent
- IFU/Operation manual
- Photo/label/packaging/UDI info (For Class-II & Class-III)
- Truth and Accuracy Statement
- Free Sale Certificate issued by the health authority by the country of origin (for imported products) and authorization letter issued by the manufacturer.
- Copies of preclinical tests, quality control procedures, and test reports
- Literature Review (Class-III IVDs and New products only).
- Clinical Investigation Reports (Class-III IVDs and New products only).
- Radioactive safety information (if applicable)
Documentation must be provided in traditional Chinese or English.
Documents required for labeling
General labeling requirements for all device classes are required to specify information on
- Product name
- Manufacturer’s name and address
- Device specifications and model number
- Intended use or indications for use
- Date of manufacture or expiration date (if applicable)
- Storage conditions (if applicable)
- Instructions for use and any necessary precautions or warnings
- Labeling should be in Chinese
In addition to the mentioned earlier, the requirements listed below are for Class-II, Class-III, and IVD medical devices.
- Product specifications, including technical parameters and performance characteristics
- Detailed instructions for use and any necessary specific warnings, biohazard symbols, pictograms, electrical safety symbols, or radiation warning signs
- Identification of the product as a medical device
- Usage limitations and contraindications
- Batch or lot number
Taiwan medical device approval process
The following are the steps involved in Taiwan’s medical device registration process:
- Utilize the TFDA database to identify your device’s classification.
- Select a Taiwan Agent to manage your device registration on your behalf.
- Prepare a quality system documentation (QSD) application unless your device is exempt. Submit the application and pay the applicable fee. Upon reviewing the documentation, TFDA will issue a QSD certificate.
- Compile the registration application/dossier for submission to TFDA along with the application fee.
- After reviewing the application, TFDA may request additional details.
- Once TFDA approves your application, a registration license will be issued.
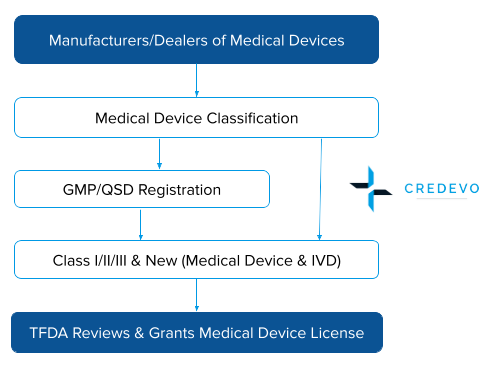
Flowchart illustrating the Taiwan Medical Device Regulatory Approval Process
Timeframe for medical device registration in Taiwan
| Class I | Class I (Sterile/Measuring) | Class II | Class III | New (No Predicate) | |
|---|---|---|---|---|---|
| TFDA Application Timeline | 1-2 Months | 7-8 Months | 8-12 Months | 9-14 Months | 10-15 Months |
| QSD Timeline | N/A | 6 Months | 6-8 Months | 6-8 Months | 6-8 Months |
Fees for medical device registration in Taiwan
| Class I | Class I (Sterile/Measuring) | Class II | Class III | New (No Predicate) | |
|---|---|---|---|---|---|
| TFDA Application Fee | NT$ 15,000(US$536) | NT$ 15,000(US$536) | NT$ 60,000(US$1,900) | NT$ 100,000(US$3,571) | NT$ 130,000(US$4,100) |
| QSD Application Fee | N/A | NT$ 60,000(US$1,900) | NT$ 60,000(US$1,900) | NT$ 60,000(US$1,900) | NT$ 60,000(US$1,900) |
Validity of licenses
Medical device registrations in Taiwan typically last five years. The QSD certificate is valid for three years. It is recommended that you submit your next renewal six months before the certificate expires.
Import license
Following approval, the TFDA grants the device license (Medical Device Permit License) to the manufacturer, and it is valid for five years. The renewal application must be filed 10 months before the expiry date.
Do You Have Any Questions In Registering Your Medical Device In Taiwan?
Do you have any questions or need assistance in registering your medical device in Taiwan or any other country in the Asia-Pacific region? Please provide your requirements in the form below to connect with us and discuss your regulatory needs.