Clinical Trial Investigators & Sites In The Asia-Pacific Region
Comprehending the function of clinical trial investigators and the accessibility of appropriate trial sites is essential, as it signifies the commencement of the market availability of your healthcare product following a successful clinical trial.

The Asia-Pacific region is gaining prominence in the world of healthcare products. The healthcare market in the Asia-Pacific region is expected to grow at a compound annual growth rate (CAGR) of 9.14%, reaching a value of USD 84.64 billion by 2029.
By delving into the characteristics of investigators, the challenges in recruiting them, and the diversity of clinical trial sites available, this article aims to provide insights into the landscape of clinical trial investigators and sites in the Asia-Pacific region, offering valuable information for companies planning to market health products in this dynamic market.
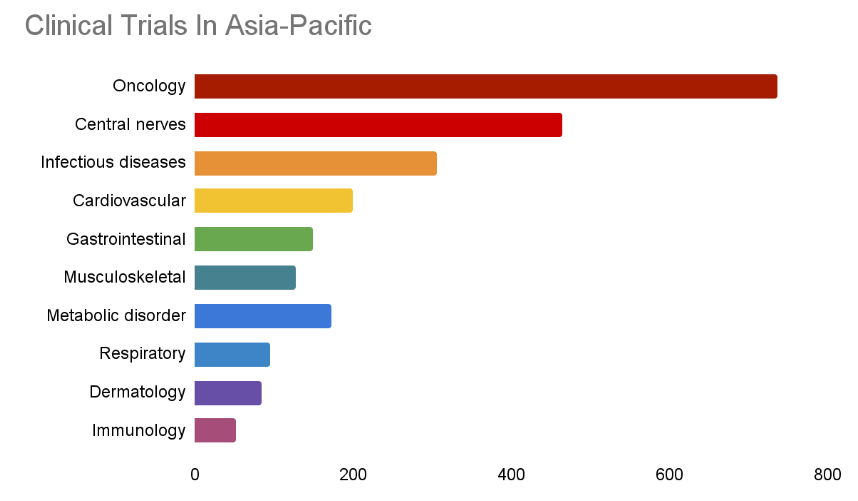
Statistics of clinical trials in the main therapeutic areas in the Asia-Pacific region
- Clinical trials in the Asia-Pacific region have experienced exponential growth, with the number of trials conducted annually steadily increasing.
- According to recent statistics, the region accounts for a significant proportion of global clinical trials, with China, Japan, South Korea, and India emerging as key players.
- The main therapeutic areas of focus in these trials include oncology, cardiovascular diseases, infectious diseases (such as COVID-19), metabolic disorders (such as diabetes), and central nervous system disorders (such as Alzheimer’s disease and depression).
Click here to gain strategic insights for choosing the Asia-Pacific region for your clinical trials.
The following chart explains the main therapeutic areas and percentage of clinical trials conducted in the Asia-Pacific region in 2023.
Top 5 reasons to choose the Asia-Pacific region for your clinical trial
Choosing the Asia-Pacific region for your clinical trial offers several compelling advantages. First, it provides access to a diverse population, enhancing the study’s relevance and applicability. These countries often boast streamlined regulatory processes, speeding up approval timelines. Lower operational costs can be found in many countries, making trials more cost-effective. A few points are discussed below out of many.
- Large patient pool
- Efficient recruitment
- Cost-effectiveness
- Regulatory framework
- Emerging markets
Role of investigators in clinical trials
Clinical trial investigators in the Asia-Pacific region play a significant role in conducting clinical research. They are typically experienced medical professionals, such as doctors or researchers, who oversee the clinical trial process. Their responsibilities include:
- Protocol development: Investigators help design the study protocol, outlining the objectives, methodology, and procedures for the clinical trial.
- Participant recruitment: They identify and recruit eligible participants for the trial, ensuring that they meet the inclusion criteria and understand the study requirements.
- Informed consent: Investigators obtain informed consent from participants, explaining the purpose of the study, potential risks and benefits, and the participant’s rights.
- Study conduct: Investigators oversee the implementation of the clinical trial, including data collection, monitoring participant safety, and ensuring adherence to the protocol.
- Data analysis: They collaborate with biostatisticians and other researchers to analyze the trial data and interpret the results.
- Regulatory compliance: Investigators ensure that the clinical trial complies with local regulatory requirements and ethical standards.
- Reporting: Investigators are responsible for reporting study findings to regulatory authorities, scientific journals, and other stakeholders.
In the Asia-Pacific region, clinical trial investigators contribute to advancing medical knowledge and improving healthcare by participating in a wide range of clinical research studies.
What are the positives of Clinical trial investigators & sites in the Asia-Pacific region?
Clinical trial investigators and sites in the Asia-Pacific region offer several advantages that make them attractive for conducting clinical research:
- Large and Diverse Patient Populations: The Asia-Pacific region is home to a diverse population representing various ethnicities, ages, and medical conditions. This diversity allows for more comprehensive clinical trials that can better assess the safety and efficacy of treatments across different demographic groups.
- Rapid Patient Recruitment: The large population density in many parts of the Asia-Pacific region enables rapid recruitment of participants for clinical trials. This can accelerate the pace of research and reduce the time and costs associated with patient enrollment.
- Access to Treatment-Naive Populations: In some cases, certain regions in the Asia-Pacific may have a higher prevalence of untreated or under-treated medical conditions, providing access to treatment-naive populations for clinical trials. This can offer valuable insights into the effectiveness of new therapies in real-world settings.
- Advanced Healthcare Infrastructure: Many countries in the Asia-Pacific region have made significant investments in healthcare infrastructure, including modern hospitals, research facilities, and clinical trial centers. This infrastructure supports high-quality clinical research and ensures compliance with international standards.
- Experienced Investigator Base: The Asia-Pacific region is home to a growing number of highly skilled and experienced clinical trial investigators. These investigators have expertise in various therapeutic areas and are well-equipped to conduct rigorous research in compliance with regulatory requirements.
- Collaboration Opportunities: Collaboration between local investigators, research institutions, industry sponsors, and international organizations is common in the Asia-Pacific region. This collaborative approach fosters knowledge sharing, capacity building, and the development of innovative research methodologies.
- Cost Efficiency: Conducting clinical trials in the Asia-Pacific region can be cost-effective compared to some Western countries. Factors such as lower labor costs, reduced administrative expenses, and competitive pricing for services contribute to cost efficiency.
- Regulatory Harmonization Efforts: Efforts to harmonize regulatory requirements across the Asia-Pacific region, such as the Association of Southeast Asian Nations (ASEAN) Common Technical Requirements, aim to streamline the clinical trial approval process and facilitate multinational studies.
Overall, clinical trial investigators and sites in the Asia-Pacific region offer numerous advantages that make them valuable contributors to the global clinical research landscape. These advantages include access to diverse patient populations, rapid recruitment capabilities, advanced infrastructure, experienced investigators, collaboration opportunities, cost efficiency, and regulatory harmonization efforts.
Here are some challenges and ways to overcome these challenges of Clinical trial investigators & sites in the Asia-pacific region
While there are many advantages to conducting clinical trials in the Asia-Pacific region, there are also several challenges that investigators and sites may encounter. Here are some common challenges and strategies to overcome them:
Regulatory Hurdles:
Variability in regulatory requirements across countries in the Asia-Pacific region can create challenges for obtaining approvals to conduct clinical trials. Additionally, navigating the regulatory landscape can be complex and time-consuming.Strategy: Engage with local regulatory authorities early in the planning process, seek expert guidance on regulatory requirements, and leverage resources such as regional regulatory harmonization initiatives to streamline approval processes.
Language and Cultural Barriers:
Language differences and cultural nuances may pose communication challenges between sponsors, investigators, and participants. Misinterpretation of study materials and informed consent documents can impact trial integrity. Strategy: Utilize qualified translators for study documents, provide culturally sensitive training to study staff, and ensure that informed consent procedures are conducted in the participant’s preferred language with appropriate cultural considerations.
Quality Assurance and Data Integrity:
Ensuring data quality and integrity can be challenging in regions where standards for Good Clinical Practice (GCP) may vary or be less rigorously enforced. Inadequate training and monitoring of study staff can compromise data quality. Strategy: Implement robust training programs for investigators and study staff on GCP principles and protocol requirements. Conduct regular site monitoring visits and implement centralized data monitoring strategies to detect and address data discrepancies proactively.
Patient Recruitment and Retention:
Despite the large population size in the Asia-Pacific region, recruiting and retaining participants for clinical trials can be challenging due to factors such as patient awareness, access to healthcare, and competing priorities.Strategy: Develop targeted recruitment strategies tailored to the local context, leverage community outreach programs and patient advocacy groups, and offer incentives or reimbursements to enhance participant engagement and retention.
Infrastructure and Resource Limitations:
While many countries in the Asia-Pacific region have advanced healthcare infrastructure, some areas may lack adequate facilities, equipment, and trained personnel to support clinical research.Strategy: Invest in capacity-building initiatives to strengthen research infrastructure, provide training and mentorship opportunities for local investigators and study staff, and consider establishing partnerships with academic institutions and research organizations to leverage resources.
Ethical Considerations:
Ensuring ethical conduct and protection of participant rights in clinical trials is paramount. Ethical challenges may arise due to differences in cultural norms, socioeconomic factors, and access to healthcare.Strategy: Establish Institutional Review Boards (IRBs) or Ethics Committees to review and approve study protocols, ensure that informed consent processes are transparent and culturally sensitive, and prioritize participant welfare throughout the research process.
By proactively addressing these challenges and implementing appropriate strategies, clinical trial investigators and sites in the Asia-Pacific region can enhance the conduct of clinical research and contribute to the advancement of medical knowledge and patient care.
Regulatory Environment
A mix of national regulations, international guidelines, and local nuances characterizes the regulatory environment governing clinical trials in the Asia-Pacific region. Regulatory bodies, such as the China National Medical Products Administration (NMPA), Japan Pharmaceuticals and Medical Devices Agency (PMDA), and Indian Council of Medical Research (ICMR), oversee the ethical conduct, safety monitoring, and data integrity of clinical research within their respective jurisdictions.
Follow the link below for more information about regulatory authority for Asia-Pacific countries.
Conclusion
In the Asia-Pacific region, clinical trial investigators and sites offer diverse opportunities but face challenges. With a growing number of trials and a focus on main therapeutic areas like oncology and infectious diseases, the region is a key market. Overcoming challenges such as regulatory complexity and logistical barriers is crucial. Efficient recruitment, cost-effectiveness, and a supportive research environment make Asia-Pacific an ideal destination for impactful clinical research.
Do you have more questions regarding clinical trial investigators and sites in the Asia-Pacific countries?
Contact one of our experts to get more details