How To Register A Prescription Medicine In Australia?

The registration of prescription medicine in Australia is straightforward, and the TGA evaluates applications for quality, safety, and efficacy. Australia ranks among the largest healthcare markets worldwide as its health system is one of the best in the world and provides quality, safe, and affordable healthcare for its citizens.

The value of the Australian pharmaceutical market was USD 25,250 million in 2020, and analysts expect it to reach USD 28,750 million in 2026, witnessing a 2.1% CAGR growth over the period.
The crucial factors likely to drive the market growth during the forecast period are
- the growing burden of chronic diseases and
- the rising geriatric population, along with
- the increasing investments in research and development expenditure for novel therapeutics in Australia.
Regulatory authority for prescription medicine in Australia
In Australia, the governing body for medicinal and pharmaceutical products is the ‘Therapeutic Goods Administration (TGA).
Classification of pharmaceuticals
TGA does the classification of medicines as per risk and potential for misuse mentioned in schedules,
Medicines can be classified as
- Prescription medicines
- Non-prescription medicines i.e. over counter medicines (OTC)
- Complementary medicines.
- Generic medicines
- Vaccines
- Biologics & Biosimilars
Note that these medicines should be included in the Australian Register of Therapeutic Goods (ARTG).
Registration process for prescription medicine
The application for registration of prescription medicines shall include non-clinical, clinical, and/or bioequivalence data (category 1 and category 2).
The regulatory process for prescription medicine
The TGA registration process has eight well-defined steps with designated milestones and an established timeframe. This stepwise process allows to plan and track applications effectively by applicant and TGA.
These steps include
- Pre-submission
- Submission
- First Round Assessment
- Consolidated section 31 request response
- Second round assessment
- Advisory Committee review
- Decision
- Post decision
It is necessary to finish the preceding step before commencing the next step. This approach allows effective and transparent management of resources and timelines for all applications.
TGA reviews all applications received in a given intake slot in one group and processes that particular group through the phases and milestones. It is known as batch processing.
Let’s discuss each step in detail.
Following are the steps for registering prescription medicine
Step 1: Pre-submission
The Pre-submission begins with lodging the Pre-submission Planning Form (PPF) and concludes when TGA sends a Planning Letter to the applicant. This step applies to Category I and Category II applications.
Submission of PPF
- The applicant should submit planning data in the Pre-Submission planning form (PPF) to TGA.
- It includes
- information about the scope and scale of the application,
- the proposed application type, and details of the quality, nonclinical, and clinical evidence that will be present in the dossier.
- It is a two-step process that uses the TGA’s secure eBS portal.
- The TGA encourages the attachment of a complete CTD Module 2.
TGA processing
TGA processes all the PPFs received on the first day of each month. It verifies all the forms if the PPF is complete and acceptable. Then the application will receive a Planning Letter.
Planning letter
It contains the date on which the applicant shall submit the dossier and the expected dates for the milestones of the regulatory process. It also mentions any issue the TGA has identified while evaluating the application.
Following the month the TGA processes the PPF, it sends the planning letter to the applicant before or on the fifteenth day of the month.
Step 2: Submission of dossier for prescription medicine
Submission begins with the receipt of the dossier and concludes with the applicant receiving a Notification letter from the TGA.
Dossier submission
At the Submission phase, the applicant company will provide a dossier, which consists of references of necessary data that states the quality, safety, and efficacy of prescription medicine, for evaluation by electronically lodging their application through eBS prior.
Applicants must ensure that the dossier meets TGA regulatory requirements for formats and submit the required dossier or application by the date mentioned in the Planning letter.
Notification letter
Before the end of the month and in the month in which the applicant has lodged the dossier), TGA issues a notification letter to the applicant.
This notification letter informs the applicant whether the application has been accepted.
- Effective and acceptable for evaluation, or
- The application is not acceptable for evaluation. It also specifies the reason for not accepting the application.
The application has to pay a full evaluation fee if the TGA accepts the application for evaluation. The notification letter will specify the charges. If not paid within two months from the date of the Notification letter, the application will lapse.
If TGA does not receive the dossier in the expected time, then the application fee is forfeited, and the application process is to begin through a new PPF.
Step 3: First round assessment
The first round assessment step will commence the day after sending the notification letter to the Applicant.
- The evaluators of TGA review and evaluate all the data provided in the dossiers.
- After evaluation of the data, If there are any issues or questions about the application, TGA complies, checks, and authorizes a consolidated section 31 request for information or documents. It issues the first round assessment within one month and provides it to the applicant.
- Period of evaluation: The designated timeframes for the first round of evaluation are
- 3 months for evaluation of new generic medicine application
- 4 months for the evaluation of other applications.
- Report: The authority sends a letter to the applicant that will include a consolidated section 31 request of information or documents OR a copy of the first round assessment reports prepared by the quality, nonclinical, clinical, and RMP
Step 4: Consolidated section 31 request response
- It allows time for the applicant to consider TGA’s consolidated section 31 request for information or documents, prepare a response and submit it to TGA.
- The section 31 response time will be either 30 or 60 days, as specified by the applicant in the PPF form and confirmed by the TGA.
- If the applicant fails to respond within the nominated period, the second round assessment will be concluded based on the information available with TGA.
Step 5: Second round assessment
On completion of the consolidated section 31 request-response phase, the second round assessment phase will commence, whether or not the TGA receives a response from the applicant.
After the second round assessment, TGA issues the second round assessment Evaluation report.
- For new generic drug applications within 2 months
- For all other applications within 1 month.
Through the evaluation report, TGA informs the applicant whether
- The TGA refers the application to the Advisory committee. Then applicants will have 13 working days before the meeting to review the Evaluation report and notify TGA of any perceived error of facts or omission.
- The TGA does not refer the application to the Advisory committee. Then applicants will have 14 calendar days to review the Evaluation report and notify TGA of any perceived error of facts or omission.
Step 6: Advisory committee review
The Advisory Committee on Medicine (ACM) is the crucial advisory body for prescription medicine. The committee may seek advice from independent experts outside the committee.
When the applicant receives notification via Evaluation report to refer to the advisory committee, this step starts and ends when the committee sends the required advice to the applicant.
The minutes of the ACM meeting advice are documented and provided to the applicant and delegates in the following meeting, which is scheduled 10 working days after the ACM meeting.
Step 7: Decision
A TGA decides on a new registration or a variation to a registration application and sends the decision letter to the applicant.
- After reviewing all documentation associated with the application till this stage of the process, delegates make risks, and benefits assessments associated with the product
- Applicants will have 14 calendar days after the TGA issues the final evaluation report(s) to review and advise the TGA of any perceived errors of fact or major omissions.
- If the benefits associated outnumber the risk associated with the product, then a letter of approval of a new registration (with possible variation or modification in the product).
- The delegate may decide to reject an application and will send a formal statement stating the reasons to reject the application.
Step 8: Post decision
The step commences when the TGA conveys its decision to the application and ends following the delegate decision.
- A new ARTG (Australian Register of Therapeutic Goods) registration can only happen when the applicant provides a patent certificate or notice.
- TGA will check the provisional ARTG record to match with the approved product description.
- The provisional ARTG record will become the ARTG Record of Registration.
- Applicant should also notify the TGA of the actual date of marketing commencement, and applicant should also fulfill other requirements mentioned in the decision letter.
- The medical information about the product and the decision should be made public via the TGA website.
Prescription medicine registration process flow
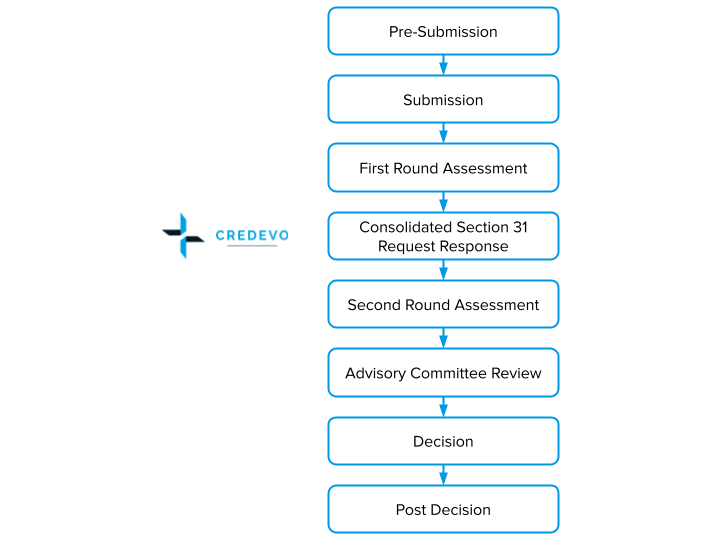
Steps in the prescription medicine registration process
Do you have any questions regarding the approval of prescription medicine in Australia?
Let us know your queries about the registration of prescription medicines in Australia. Credevo offers services in registering medical devices, complementary medicines, and prescription products in Australia. Submit your requirement details below to connect with us and explore our services.