Regulatory Approval For Nutraceuticals (Complementary Medicines) In Australia Part-I

Do you wish to market your nutraceuticals/complementary medicines (vitamins, minerals, nutraceutical products, dietary supplements, and herbal products) in the Australian market? Then it is better to realize that the Australian regulatory requirements are different from other countries for such products.

For example, in the United States of America (USA), by filing the GRAS status (Generally Recognized As Safe), such products can reach the pharmacy store, supermarkets, and retail stores … along with, of course, some basic documentation. But it’s a lot easier there.
The market in Australia is divided into three segments such as dietary supplements, functional food, and functional beverages.
The Food Standards Australia New Zealand (FSANZ) is a statutory authority in the Australian Government health portfolio that develops food standards for Australia and New Zealand.
On the other hand, Therapeutic Goods Administration (TGA) conducts a thorough review and evaluates dietary supplement products for safety, efficacy, and quality before the products reach the Australian market and consumers.
While other countries may classify these products as
- Herbs,
- Vitamins,
- Minerals,
- Nutritional supplements,
- Homeopathic medicines,
- Traditional medicines,
- Ayurvedic medicines, or
- Aromatherapy, in the case of certain other products
But not Australia!
For the Australian agency, TGA, the name is medicines, “Complementary Medicines”
So, what is the process for registering complementary medicines in Australia?
How to register Complementary medicines (vitamins, minerals, dietary supplements, or herbal products) in Australia?
Let’s understand how TGA defines complementary medicines.
What are the Complementary Medicines in Australia?
As per the TGA, Complementary Medicine means a therapeutic good consisting wholly or principally of one or more designated active ingredients, each of which has an established identity and traditional use.
The salient points to consider are as follows.
- TGA regulates complementary medicines under the Therapeutic Goods Act (1989).
- These are generally available for use in self-medication by consumers.
- The majority of complementary medicines are indicated for the relief of symptoms of minor, self-limiting conditions, maintaining health and well-being, or the promotion or enhancement of health.
Australian regulatory body TGA has developed the Australian Regulatory Guidelines for Complementary Medicines (ARGCM) to assist sponsors of complementary medicines to meet their legislative obligations.
One can obtain detailed information by visiting the TGA website.
Need support to register and market your vitamins, minerals, nutraceutical, dietary supplement, herbal products, or any other product in Australia?
Credevo provides complete regulatory support for registering your complementary medicines and pharmaceutical product in Australia. Fill out your details in the below form and talk to us now.
Regulatory aspects for filing complementary medicine/nutraceuticals in Australia
- The sponsor needs to list or register the complementary medicines on the Australian Register of Therapeutic Goods (ARTG).
- ARTG maintains a database that includes details of all therapeutic goods imported into, supplied in, or exported from Australia.
- Based on the risk associated, each therapeutic good gets an AUST number. All marketed products must contain the AUST number on their product label with the sponsor’s name and address.
- It is a legal requirement that, unless exempted or excluded, all therapeutic goods are included on the ARTG before their Supply to the consumer.
- An applicant shall submit a detailed dossier of information to the TGA for evaluation. This data must establish that the proposed medicine is of appropriate quality, safety, and efficacy for approval and inclusion on the ARTG.
TGA Classification for complementary medicines
Complementary medicines may either be
- Listed or
- Registered
depending on their ingredients and the therapeutic claims made for the product.
- The therapeutic claims must have evidence. The stronger the claims made, the stronger the evidence must be.
- Australian TGA adopts a risk-based approach for regulating their medicines review.
- Low-risk products are regulated as ‘Listed Medicines’ compared to high-risk products, which need to be evaluated and marked as ‘Registered Medicines’
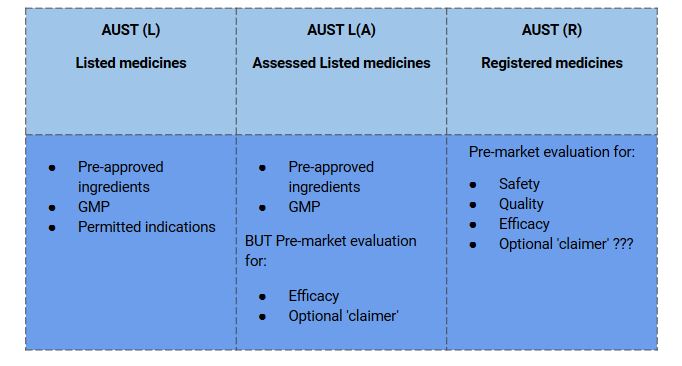
- Let’s understand, what are the parameters the TGA monitors for listed and registered medicines through the table below.
Listed Medicines
Listed medicines are usually considered to be relatively benign, so the regulations allow for sponsors to ‘self-assess’ their products in some situations. The majority of listed medicines are self-selected by consumers and used for self-treatment.
- Listed medicines are ‘low-risk’ medicines.
- It contains certain low-risk ingredients in acceptable amounts permitted for use in listed medicines by the TGA.
- The product must be manufactured by the principles of Good Manufacturing Practice (GMP)
- The manufacturing of products must be by the principles of Good Manufacturing Practice (GMP).
- The indications/claims on listed medicines are not subject to pre-market evaluation. However, at the time of Listing, the Act requires that sponsors certify that they hold the evidence to support indications and claims and that claims are true, valid, and not misleading.
Assisted Listed Medicines
- Medicines listed in the ARTG through the assessed listed medicines pathway follow self-certification of the safety and quality of the product.
- TGA performs a pre-market assessment of efficacy to support the proposed indications.
Registered Medicines
- Based on their ingredients or the indications made for the medicine, Registered complementary medicines are at relatively higher risk than listed medicines.
- TGA evaluates the registered medicine completely for quality, safety, and efficacy before accepting them on the Australian Register of Therapeutic Goods (ARTG) and marketing.
Based on the product category decided by you, for your product submission to TGA, you have to prepare a comprehensive dossier for regulatory approval.
Are you planning to register & market your Nutraceutical product (Complementary Medicine) in Australia?
Talk to us today. Provide preliminary details below, and we will help you achieve a successful registration in Australia. Credevo can be your strategic partner to make a successful submission on time.
You will get a complete overview of the regulatory framework, process overview, and submission timelines.
To prepare the dossier for TGA submission, one must consider 6 important aspects related to that. There are crucial requirements related to these sections that we have discussed in part II of this series.
Check out part II of this article for more information →
Reference
1. https://www.tga.gov.au/complementary-medicines
2. https://www.tga.gov.au/overview-regulation-complementary-medicines-australia
2 thoughts on “Regulatory Approval For Nutraceuticals (Complementary Medicines) In Australia Part-I”
Comments are closed.