Generic Drug Registration Process In Malaysia

There is a growing demand for generic drugs in Malaysia. The government is the largest pharmaceutical buyer in Malaysia and is responsible for more than half of the purchasing value. The Indian, Chinese, and other similar drug manufacturers have begun playing a dominant role in the Malaysian pharmaceutical market.
Note: This article was updated in September 2022.

In Malaysia, the pharmaceutical manufacturing sector produces exclusively generic drugs, and the sales revenues amount to only 1.5% of the gross domestic product (GDP). That shows the great demand for generic medicines in Malaysia.
This demand is due to the steep rise in non-communicable diseases, and some other reasons include,
- With the increase in the prevalence of unhealthy diets,
- Increasing sedentary lifestyles, and
- Use of tobacco and alcohol.
Malaysia has one of the highest rates of diabetes in the Asia-Pacific, with almost one-fifth of its population diagnosed with the disease.
Other most found health complications in the Malaysian population include
- Obesity
- Hypertension and heart diseases
- Stoke
- Respiratory disease
- Cancer
Definition of generic drug in Malaysia
A generic product is a pharmaceutical product similar to a currently registered product in Malaysia. However, the term generic drug does not apply to Biologics.
Types of generic drugs in Malaysia
Generics are classified into two groups
- Scheduled Poison (Known as Controlled Medicine/ Controlled Poison).
- Products containing poisons as listed in the First Schedule under the Poisons Act 1952.
- Non-scheduled Poison (Known as Non-Poison or “Over-the-Counter” OTC).
- Products containing active ingredients that are not listed in the First Schedule under the Poisons Act 1952; and excluding active ingredient which is categorized under health supplements natural products or cosmetics.
Learn about the pharmaceutical registration process in Malaysia
The regulatory authority for generic drug registration in Malaysia
The National Pharmaceutical Regulatory Agency (NPRA), formerly known as the National Pharmaceutical Control Bureau (NPCB), is the main regulatory body for pharmaceutical products in the country. The NPRA is an organization under the Pharmaceutical Services Division (PSD) of the Ministry of Health (MoH).
The Drug Control Authority (DCA) is the executive branch of the NPRA and is responsible for drug registration, licensing for importers, and manufacturers, and drug quality monitoring.
What are various types of generic drug application evaluation processes?
Generic pharmaceutical products
| S. No. | Product Category | Full Evaluation | Abridged Evaluation |
| 1 | Generics (Scheduled Poison) | Yes | -NA- |
| 2 | Generics (Non-scheduled Poison) [or known as OTC] | All products from this category, unless stated in Abridged Evaluation | Includes, but is not limited to the following 1. Antiseptics/skin disinfectants 2. Locally-acting lozenges/ pastilles 3. Topical-analgesics/counter-irritants; 4. Emollient/demulcent/skin protectants, etc. 5. Topical-nasal decongestants |
Abridge evaluation process
The Abridge review process is simple when compared with a full evaluation. This category is exempted from certain information in Part I ACTD and Process Validation Report. The Abridged Evaluation does not require Quality Control pre-registration documentation such as the protocol of analysis (PoA) and analytical method validation (AMV) report.
Abridged Evaluation is for low-risk pharmaceutical products based on these 3 factors
- Active ingredient – contains well-established active ingredients
- Dosage form and route of administration: external preparations and locally acting dosage form
- Indication: used for non-critical conditions only (e.g.: acne, dandruff, counterirritant, antiseptics).
Need support for your drug registration in Malaysia?
Credevo offers expertise in drug product registration, clinical trial regulations, and many more services in Malaysia. Check them out now!
Bioavailability (BA)/Bioequivalence (BE) studies
Malaysia has fully adopted the ASEAN guideline since March 2015, and it is the most current guide for the conduct of bioequivalence studies. This guideline is adopted from the Guideline on the Investigation of Bioequivalence” (European Medicines Agency, London, 20 January 2010, CPMP/EWP/QWP/1401/98 Rev 1) with some adaptation for ASEAN application.
- Some categories of products require Bioavailability (BA) studies. For example, modified-release products.
- Bioequivalence Studies are required for the drug products falling under the category of scheduled poison generics.
- There are some exemptions for submitting BA/BE studies, provided by fulfilling the criteria set by the regulators.
Manufacturing site requirements for generic drug registration
- Compliance with Good Manufacturing Practice (GMP) is a prerequisite to the application of generic product registration.
- It is to ensure that the products manufactured are safe, have efficacy, and are of quality.
- Pharmaceutical Inspection Co-operation Scheme (PIC/S) Guide to Good Manufacturing Practice (GMP) for medicinal products and its annexes have been adopted as the standard used by NPCB to assess the GMP conformity of manufacturers.
Requirements for foreign manufacturers to register the generic drug in Malaysia
Manufacturers need to comply with the standards of manufacture and quality control for medicinal products manufactured outside Malaysia before they register with the authority namely Drug Control Authority (DCA).
One of the requirements to register an imported medicinal product in Malaysia is the submission of acceptable evidence on the GMP compliance of the manufacturer in a prescribed format.
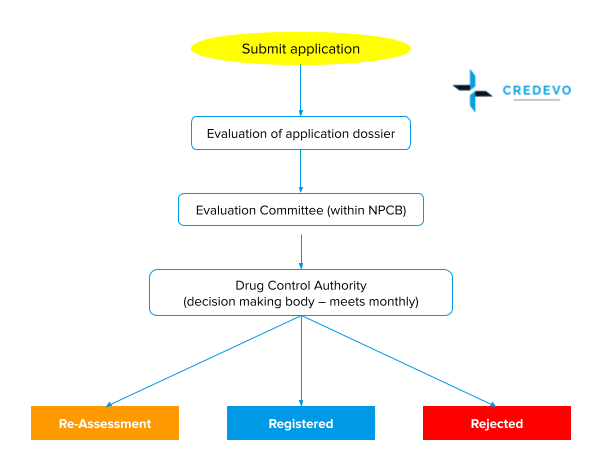
The drug registration process in Malaysia
- The company must register with the Malaysian Registrar of Business (ROB) or the Companies Commission of Malaysia (SSM).
- Applicants need to submit drug applications through a web system to the DCA.
- The applicant must submit the required documents to support an application for product registration following ACTD guidelines.
- The evaluation committee with NPCB will evaluate the application and may
- Accept
- Reject
- Order for Re-assessment
- Upon approval during screening, the applicant is requested to proceed with payment and submission of hard copy documents.
Review fees for generic applications
The fee for review of the various generic drug products is approximately between RM 2,200.00 to RM 3,000.00.
Timelines for generic applications review
The timeline for drug approval is
- The standard route has a target time of 365 days,
- The abbreviated route has a target timeline of 180 days, and
- The verification route has a target of 135 days.
Post-approval distribution process
- Any company that wishes to manufacture, import, and/or wholesale any registered product should have a Manufacturer’s Licence, Import Licence, and/or Wholesaler’s License.
- The company or sponsor needs to submit an application form with supporting documents. For the complete list of documents please click on the link below to download the report.
- The authority processes the application only if it finds that the application form complete along with the payment receipt.
- The processing fee shall not be refundable. The processing fee of an application for a Manufacturer’s License is RM1,000.00 and RM500.00 for an Import License or a Wholesaler’s License.
- Each license is valid for one year.
Do you need support or have queries on drug registration requirements?
Credevo offers a wide range of drug development and regulatory services in Malaysia. Choose one of the following options to connect with us.
Get the report on the generic drug registration process in Malaysia.
Note: This report will be charged @ $359.
Do you have a query? Just ask experts at Credevo.
Note: “Ask Credevo Expert” will be charged @ $50 / inquiry. Any inquiry requiring more than 30 min of expert’s time will incur additional charges.
Looking for a quotation? Just provide relevant info and we will send you the details.