Singapore Generic Drug Approval Process

Singapore has become a global center for the pharmaceutical industry due to its cost-effective and reliable medical services, high-quality pharmaceutical manufacturing and research, access to regional markets, and skilled workforce. Also, the Government has supported several schemes to drive innovation and development. This has created many manufacturers to register their generic products in Singapore.

The growing healthcare demand and the adoption of modern technology are driving Singapore’s pharmaceutical market further. Singapore invests a huge amount in the development of the healthcare system, and Singapore’s healthcare system holds the sixth position worldwide as per WHO. Singapore has well-established pharmaceutical regulations among Asian countries.
The Singaporean pharmaceutical market is estimated to grow at a CAGR of 6.40% for the next ten years, and the total revenues of the Singaporean generics market were $0.4 billion in 2019. In 2019, about 31.4% market share of prescription medicines in Singapore was generic drugs, and is expected to reach 38.9% and about 567 million U.S. dollars by 2027.
Let’s understand the regulations governing generic drugs for registration in Singapore.
What is a Generic drug in Singapore?
A generic drug application applies to therapeutic products that contain one or more chemical entities and is essentially the same as a Singapore Reference product in terms of its qualitative and quantitative composition of active ingredients.
Regulatory for Generic drug registration in Singapore
In Singapore, the Health Sciences Authority (HSA) is the regulatory authority for regulating pharmaceutical product registration.
Who can apply for registration?
To proceed with therapeutic product registration in Singapore, a local company must be registered with the Accounting and Corporate Regulatory Authority (ACRA).
Need support for your drug registration in Singapore?
Credevo offers expertise in drug product registration, clinical trial regulations, and many more services in Singapore. Check them out now!
Generic Drug Application (GDA)
The applicant can register the generic drug if the drug meets the following criteria:
- The same pharmaceutical dosage form as Singapore’s reference product.
- Same route of administration as the Singapore reference product.
- Bioequivalent with the Singapore reference product.
- Has conditions of use that fall within the directions for use (including dosing regimen, indication, and patient group) for the Singapore reference product.
Application types for generic drugs in Singapore
There are two types of generic drug applications (GDA) to register and market generic drugs in Singapore.
- GDA-1: This type of application is for submission of the first strength of a generic chemical product.
- GDA-2: This type of application is for submission of subsequent strength of the product that has been registered or submitted as GDA-1. The product name and dosage form must be the same as GDA-1.
Evaluation of generic drug application
Before applying for generic drug approval in Singapore, one needs to understand the requirements and regulations governing different evaluation routes for generic drugs.
There are three types of evaluation routes for generic drug registration in Singapore and are as follows
- Abridged evaluation route
- Verification evaluation route
- Verification evaluation route (CECA Scheme)
Application dossier format
The application for generic drugs shall be in the International Council for Harmonisation Common Technical Document (ICH CTD) or ASEAN CTD (ACTD) format. The application shall be in the English language.
How to submit a Generic drug application?
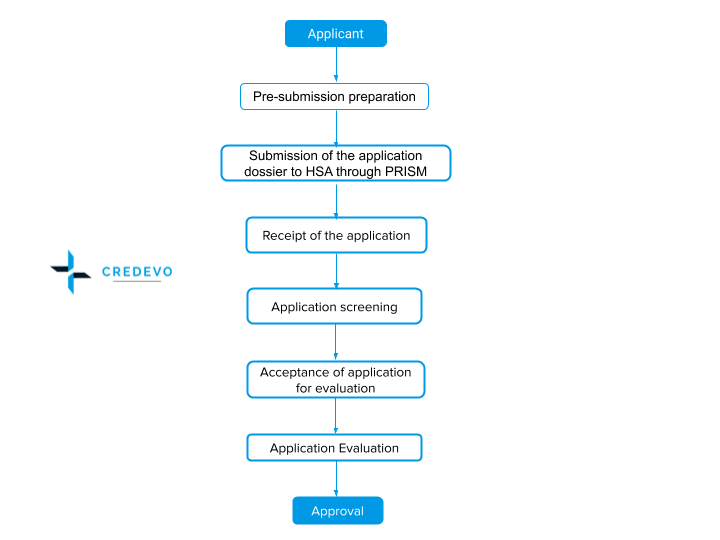
The applicant can submit the application through an online platform PRISM (Pharmaceutical Regulatory and Information System. Submit all administrative documents under Module 1 or Part I in soft copy through PRISM, and submit other parts of the documents online via PRISM or on a CD/DVD labeled with the required information.
Timelines for screening and evaluation of GDA
- Screening time for the application through three evaluation routes takes 50 working days. Screening time starts from the date of receipt of the application dossier.
- The timeline may change if the applicant is required to provide clarification or additional information in response to insufficient queries.
Notification of the Regulatory Decision
The authority screens the application for any deficiency and upon correction and fulfillment of details. The regulatory authority accepts the application if it feels complete. The regulatory authority evaluates the application and notifies the regulatory decision as follows.
- Approval
- Approvable
- Non-approvable
- Rejection
An approval decision for any therapeutic product given by HSA implies that the application satisfies the registration requirements in quality, safety, and efficacy.
After approval, the authority includes the product in the Register of Therapeutic Products. A product registrant is responsible to ensure the product’s quality, efficacy, and safety throughout its life cycle, and submit if any post-approval changes within a specified time.
Process flow
Processing fees for generic drug applications in Singapore
- The applicant needs to pay the screening fee on submission of the application and an evaluation fee after acceptance of the application.
- The applicant needs to pay the annual retention fee per registered product to maintain the register.
Do you need support or have queries on drug registration requirements?
Credevo offers a wide range of drug development and regulatory services in Singapore. Choose one of the following options to connect with us.
Get the report on the generic drug registration process in Singapore.
Note: This report will be charged @ US$359.
Do you have a query? Just ask experts at Credevo.
Note: “Ask Credevo Expert” will be charged @ US$50 / inquiry. Any inquiry requiring more than 30 min of expert’s time will incur additional charges.
Looking for a quotation? Just provide relevant info and we will send you the details.