China Drug Registration Process

China’s pharmaceutical drug market is constantly growing and is the second-largest pharmaceutical industry in the world, after the United States. It is estimated to reach $161.8 billion by 2023, with an average growth rate of 5% in the coming years, taking a 30% share of the global market.

The acceleration of new drug access, changing regulatory landscape of individual categories, the rapidly aging Chinese society, and subsequently increasing medical needs provide a range of opportunities for the pharmaceutical market.
After 13 years, the Chinese regulatory has made several changes in the rules and guidelines for registering drugs. These changes made are to reduce the complexity and match up with the other global regulators.
Highlights of China’s new provisions and opportunities for drug registration
Let’s look at some highlights of new provisions made by the Chinese regulators
- The 60 days of timeframe for CTA approval following the global regulations.
- Clear timelines for review and approval for different types of drug registration applications.
- Streamline processes between drug registration and manufacturing authorization to ensure GMP.
- Improvement of communications between the applicant and Center for Drug Evaluation (CDE) for effective CTA process.
- The introduction of parallel risk-based site inspections and lab tests registration conduction with the technical review.
- Breakthrough and the conditional approval during the clinical trial stage, and the priority review during marketing approval expediting drug registration and satisfying unmet medical needs in China.
Need support for your drug registration in China?
Credevo offers expertise in drug product registration, clinical trial regulations, and many more services in China. Check them out now!
Regulatory Authority for drug registration in China
The National Medical Products Administration (NMPA) is the main regulatory authority responsible for drug registration management, formulating drug registration specifications, and organizing drug registration review and approval.
NMPA’s Drug Evaluation Center (CDE) is responsible for the review of drug clinical trial applications, drug marketing authorization applications, supplementary applications, and drug re-registration applications for overseas manufactured drugs.
Who can apply for drug registration in China?
The Marketing Authorization Holder (MAH) can submit applications for drug clinical trials, drug marketing authorization, re-registration, and supplementary applications. Along with these duties, MAH can also handle various other duties.
Medicinal product registration categories in China
The requirements to develop and register a medicinal product in China depend on further classification:
- Chemical medicine
- Biological products
- Traditional chinese medicine.
Each type is then classified into three registration categories, which determine the materials that the applicant must provide as part of its registration application example clinical trial application, marketing authorization application, etc.
For chemical medicines, registration categories are as follows
- Innovative drugs
- Improved new drugs
- Generics
For biologics, registration categories are as follows
- Innovative biological products
- Improved new biological products
- Marketed biological products (including biosimilars)
The corresponding application materials requirements are based on the product characteristics, degree of innovation, and review management needs of the registered medicines.
The drug category in which an applicant chooses to register decides the clinical trial application review and approval process. NMPA manages the clinical trial application.
The review process for drug marketing authorization
- After completing the pre-clinical studies and clinical studies supporting the drug registration, the applicant should submit the drug marketing authorization application according to the requirements.
- After the formal examination of the application materials, acceptance will be given if they meet the requirements.
- The Drug Evaluation Center (CDE) organizes pharmacy, medical, and other technical personnel to review the accepted drug marketing authorization applications.
- After the conclusion of the comprehensive review, issues a registration certificate for drugs. If the conclusion of the comprehensive review is not passed, the application is disapproved.
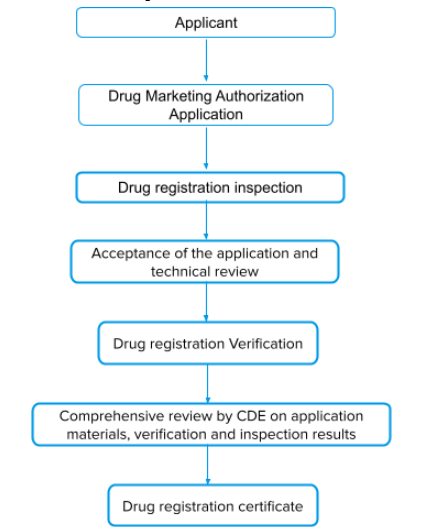
Process flow
Drug registration certificate validity and re-registration
The validity period of the drug registration certificate of drug Marketing Authorization Holder is five years. During the validity period, the MAH must take responsibility of the safety, effectiveness and quality controllability of the listed drugs and apply for drug re-registration six months prior the expiration of the validity period.
Accelerated approvals for drugs registration
According to the regulation, NMPA initiated new drug review process and has divided the approval process into the following four categories
- Breakthrough approval
- Priority review
- Conditional approval
- Special approval
The applicant needs to know for which category of drugs do these review processes apply at which stage of drug development may apply.
Registration fees for review and approval
The applicant is required to pay a fee per applicable regulations. The National Medical Products Administration (NMPA) charges the drug registration fees to review and approve clinical trials as a part of the drug registration process. The fees are different categories of drugs as below.
- New drugs made in China
- New drugs made outside China
- Generic drugs made in China
- Generic drugs made outside China
Do you need support or have queries on drug registration requirements?
Credevo offers a wide range of drug development and regulatory services in China. Choose one of the following options to connect with us.
Get the report on China drug registration process.
Note: This report will be charged @ $1424.
Do you have a query? Just ask experts at Credevo.
Note: “Ask Credevo Expert” will be charged @ $50 / inquiry. Any inquiry requiring more than 30 min of the expert’s time will incur additional charges.
Looking for a quotation? Just provide relevant info and we will send you the details.
References
- https://www.appliedclinicaltrialsonline.com/view/regulatory-requirements-and-key-points-drug-clinical-trials-registration-china
- https://www.cov.com/-/media/files/corporate/publications/2020/04/china-promulgates-revised-drug-registration-regulation.pdf
- https://www.acuritmedcomms.com/2020/04/13/china-new-provisions-for-drug-registration-2020/
- http://gkml.samr.gov.cn/nsjg/fgs/202003/t20200330_313670.html
- https://clinregs.niaid.nih.gov/country/china/united-states#scope_of_assessment
- https://www.pacificbridgemedical.com/regulatory-services/pharmaceutical/product-registration/china/
- https://www.europeanpharmaceuticalreview.com/article/98200/china-and-the-evolving-regulatory-landscape/
- https://clarivate.com/cortellis/article/regulatory-reform-in-china-enhancing-clinical-trials-review-and-approval/#:~:text=The%20applicant%20only%20needs%20three,approval%20system%20to%20tacit%20permission.
- https://globalregulatorypartners.com/chinas-nmpa-introduces-new-revised-regulation-for-drug-approval-by-foreign-companies/
- https://elc-group.com/wp-content/uploads/2020/04/RA_Drug-registration-in-China.pdf
- http://english.nmpa.gov.cn/2019-07/25/c_390595.htm
- https://globalforum.diaglobal.org/issue/may-2020/revised-drug-registration-regulation-brings-new-challenges-and-opportunities-to-new-drug-clinical-trials-in-china/
- https://pharmaboardroom.com/legal-articles/amended-drug-registration-regulation-aims-to-strengthen-and-streamline-regulation-of-new-drugs/#:~:text=On%20March%2030%2C%202020%2
- https://www.lexology.com/library/detail.aspx?g=75804955-2cd9-4c55-8654-6ca5ddcd1e34