What You Need to Know to Register Nutraceutical, Dietary, Or Food Supplement Products In Europe – Part II

Nutraceuticals or Health supplements are gaining importance and becoming a part of the consumer’s daily diet. With the increase in life expectancy and subsequent increase in lifestyle-related diseases, nutraceutical or health supplements have emerged as a necessity for consumers, especially in a developed market and Europe is not an exception to it!

Novel food represents one category among many including food supplements, nutraceuticals, nutrition, plant and animal feed. Read the first part of this article ‘What You Need to Know to Register Nutraceutical, Dietary Or Food Supplement Products In Europe – Part I‘ to learn about the Novel Food and their legislation in Europe.
Last year, the EU introduced significantly different regulations than its predecessor, and lots of new products entered the European market as Novel Food. Non-member countries are now importing exotic food plants, animals, and insect food, which were never used for human consumption in the EU before.
To market a Novel Food that has not been authorized, you need to apply for authorization. There are two authorization routes under the EU Novel Food Regulation no. 2015/2283.
In both cases, you must provide a dossier of information and submit it to the European Commission through an electronic portal. Let’s understand the Novel Food authorization process here.
Authorization procedure for Novel Food approval
Novel Food Authorization can be filed and obtained based on the pathway selected by the applicant:
- New Novel Food authorization
- Traditional food from non-country/third-country notification
In both pathways, the application is submitted electronically and the structure of the application should contain:
- Cover letter
- Technical dossier
- Summary of the dossier
The applicant may withdraw its application at any time and terminate the procedure in both pathways.
If you wish to understand the differentiation between these two pathways, read through our first blog where we have defined these two categories of Novel Food and applicable legislation for such products.
Submit the structured dossier to the European Commission for Novel Food dossier submission and notification. Discuss the requirements for each pathway in each section below.
Authorization procedure for New Novel Food
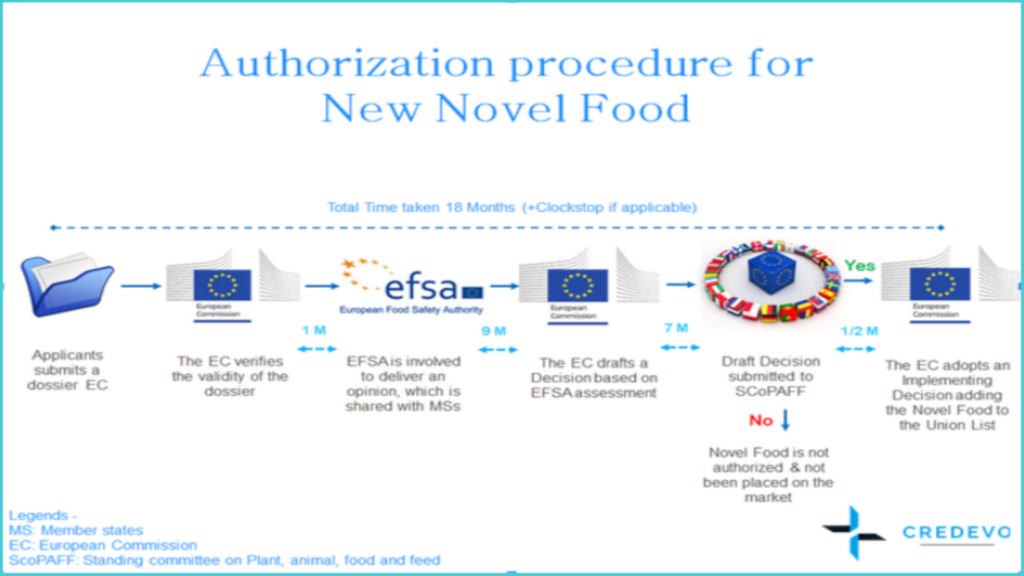
1. The Sponsor/applicant submits the product dossier to the European Commission.
2. The application for authorization shall include the following:
- Name and address of the applicant
- Name and description of the Novel Food
- Description of the production process(es)
- Detailed composition of the Novel Food
- Scientific evidence demonstrating that the Novel Food does not pose a safety risk to human health
- Analysis method(s) where applicable
- Proposal for the conditions of intended use and for specific labelling requirements which do not mislead the consumer or a verifiable justification why those elements are not necessary.
3. The European Commission verifies the validity of the application and forwards the valid application to EFSA not later than a month, from receipt of the application.
4. EFSA should provide its opinion within nine months from the date of receipt of a valid application to –
- European Commission
- Member states and,
- Applicant; where applicable
5. If EFSA requests additional information from the applicant, the nine months defined for providing its opinion can be extended.
6. After consulting with the applicant, EFSA should specify a period within which that additional information should be provided by the applicant and EFSA inform the European Commission about the extension.
7. If EFSA informs the European Commission, and there is no objection within eight working days, the European Commission will automatically extend the nine months provided for its opinion by that additional period. The Commission shall inform such extension to member states.
8. When an applicant fails to provide additional information to EFSA within the additional period provided to the applicant, EFSA shall draw up its opinion based on the available information.
9. Where an applicant submits additional information on its initiative, it shall send that information to EFSA. In such cases, EFSA should give its opinion within nine months to the European Commission.
10. Seven months from the date of publication of EFSA’s opinion, the European Commission should submit a draft commission decision to the Standing Committee on Plants, Animals, Food and Feed ( SCoPAFF) for adding/updating the Novel Food in the Union List and authorizing the placing of the Novel Food on the market, provided –
- Novel Food takes care of the underlying principle of review – i.e. safety of the food, intention of use, and product label.
- any relevant provision of Union law, including the precautionary principle (mentioned in Regulation (EC) No 178/2002 in article 7) is addressed properly.
- EFSA’s opinion is taken into consideration.
- any other legitimate factors relevant to the application under consideration.
11. In case, the European Commission has not requested the opinion from EFSA, the European Commission should provide its draft decision to the Standing Committee on Plants, Animals, Food, and Feed ( SCoPAFF) within seven months from the date of a valid application.
Notification procedure of traditional Novel Food from Non-EU country
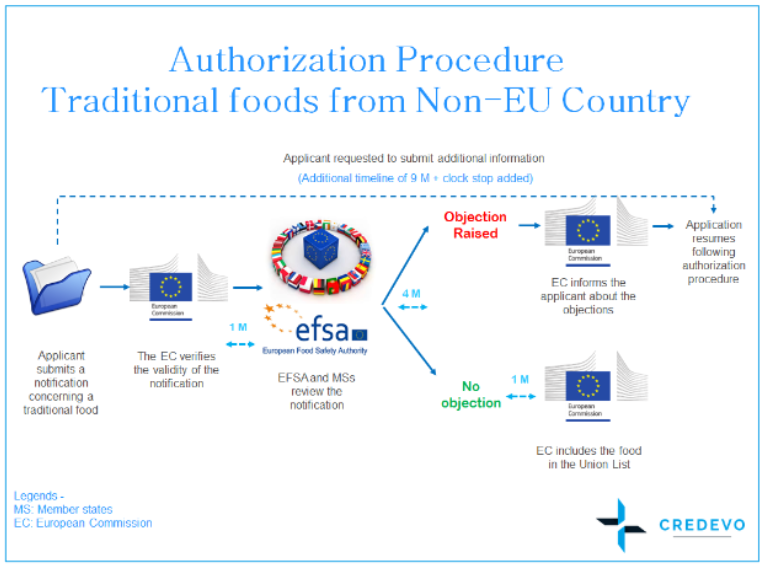
1. If a sponsor chooses to market their Novel Food product as “Traditional food from a Non-EU country /third country”, they may opt to submit a notification of that intention to the European Commission.
2. The notification shall include the following information:
- Name and address of the applicant;
- Name and description of the traditional food;
- Detailed composition of the traditional food;
- Country or countries of origin of the traditional food;
- Documented data demonstrating the history of safe food use in a third country;
- Proposal for the conditions of intended use and for specific labelling requirements, which do not mislead the consumer, or a verifiable justification why those elements are not necessary.
3. The European Commission verifies the validity of the notification and forwards the valid notification to EFSA and member states no later than a month, from receipt of the notification.
4. Within four months from the date on which the European Commission, a member state, or EFSA forwards a valid notification, they may submit duly reasoned safety objections to the European Commission for placing the traditional Novel Food on the market within the Union of the traditional food concerned.
5. The European Commission informs the applicant of any duly reasoned safety objections as soon as it is submitted. The member states, EFSA, and the applicant shall be informed of the outcome of the procedure.
6. The applicant may submit additional safety evidence to support the objection raised by EFSA or member states to the European Commission. The European Commission forwards the valid application to EFSA and member states.
7. EFSA will provide an opinion within six months from the date of receipt of a valid application to the European Commission, the member states, and the applicant.
8. In duly justified cases, where EFSA requests additional information from the applicant, the six months provided may be extended.
9. After consulting with the applicant, EFSA should specify a period within which that additional information should be provided by the applicant and EFSA should inform the European Commission of such an extension.
10.
If EFSA informs the European Commission and there is no objection within eight working days, the European Commission will automatically extend the six months provided for its opinion by that additional period.
The Commission shall inform such extension to the member states.
11. When requested additional information is not provided by the applicant to EFSA within the additional period provided, EFSA shall draw up its opinion based on the available information.
12. Where an applicant submits additional information on its initiative, it shall send that information to EFSA. In such cases, EFSA should give its opinion within six months to the European Commission.
13. If no duly reasoned safety objections are submitted within four months, the European Commission shall authorize the placing of the traditional Novel Food from a non-EU country/the third country on the market and update the Union list without delay.
14. The entry in the Union list shall specify that it concerns a traditional food from a third country/non-EU country. Where applicable, certain conditions for use, specific labeling requirements, or post-market monitoring requirements may be specified.
Maintaining Union List of Novel Food
The initial Union list was established by the European Commission implementing regulations 2017/2470, and it includes all the Novel Foods already authorized under previous regulations. Future Novel Food authorization will be managed by the European Commission by implementing its new regulations.
The European Commission can update the Union List by the following means:
- Adding a Novel Food to the Union list;
- Removing a Novel Food from the Union list
- Adding, removing, or changing the specifications, conditions of use, additional specific labeling requirements, or post-market monitoring requirements associated with the inclusion of a Novel Food in the Union list.
Post-market monitoring requirements
- The European Commission may impose post-market monitoring requirements on a case-by-case basis for food safety reasons and taking into account EFSA’s opinion.
- Sponsor/any food business operator who has placed Novel Food on the market shall immediately inform the European Commission of any information of which it has become aware concerning:
- any new scientific or technical information that might influence the evaluation of the safety of users of the Novel Food;
- any prohibition or restriction imposed by a third country in which the Novel Food is placed on the market.
- The Commission shall make that information available to the member states.
Conclusion
All in all, the European Commission has introduced simplified and centralized Novel Food authorization procedures which brings a lot of clarity and an opportunity for all the nutraceutical companies and the food business operator to boost their innovation and competitiveness without having to build an expensive and time-consuming dossier each time they want to use Novel Food which is already authorized.
Are you planning to obtain regulatory approval for your Novel Food product in Europe?
Talk to us today!
You may have a query or need a plan for European registration of your food product.
We are here to help.
Note: “Ask Credevo Experts” will be charged @ $50 / inquiry. Any inquiry requiring more than 30 min of expert’s time will incur additional charges.