How can small-mid scale bio-pharma fast track their clinical development & product registration processes? Leveraging secrets of a global network
Cost and time for clinical development constitute more than 70% of the total development of any drug! Small – mid-scale biopharma are known for their innovative approaches, agility, and faster developments. However, clinical development has always been one step too important, yet too hard for them. There are ways to overcome these challenges and optimize cost and development time. Explore more! (This article was originally published on LinkedIn).
When in Rome, do as the Romans do!
Old saying, but still holds true in the modern world.
Globalization!
A modern concept that has made services generally available at lower prices, more easily and at faster pace.
Amazing what these seemingly contradicting pair bring out, when you consider the globalized world of pharmaceutical / biopharm products.
Let me explain.
Small – medium scale pharma companies are known for their innovative approaches, agility and faster developments. In recent several years, many innovative products have been developed and brought to the market by these companies. However, clinical development has always been one step too important, yet too hard for them.
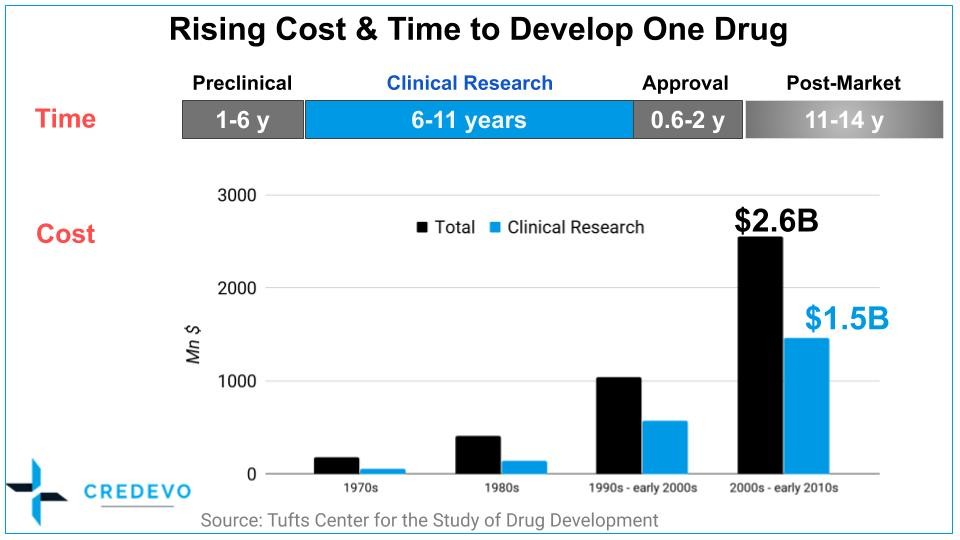
Cost and time that clinical development consumes in process of drug development is about 70% of total. What this means is, optimizing this step could work to reduce time and cost required to bring a drug to the market.
Secrets of globalization
However, the globalization has taught us that the cost and time can differ significantly across regions. And, most importantly, one should connect with teams across regions to get benefits of these differences.
Application to clinical development
In the case of clinical development too, if one factor the availability of patients (for a specific study design), expert teams, the general quality of processes, and cost of labor, it’s possible to zero on a specific region that can prove to be a better clinical development site, costing less and delivering faster.
Challenges
But, how does one work in another region, especially if they do not have their local teams in many countries (the most common situation of small-medium clinical-stage biopharma)?
Well, how about utilizing existing local teams in the region? From local service providers, local experts, and local freelancing teams.
I know, I know…
It’s not exactly “..do as the Romans do“, rather, “..get more Romans on your side“. But, you get the idea.
Getting back to ‘utilizing local clinical development teams in the region’, it could be a practical challenge to identify, connect and evaluate local teams for different conditions and regions, particularly if you have many projects, or need to work with many countries.
This is where we thought, expanding our global network of sites & investigators to become a global network of clinical development teams could be useful.
Utilizing an expanded global network for clinical development
Credevo had started as a global network of sites & investigators that is being utilized for online feasibility providing benefits of reduced cost, reduced time, and greater ease in investigator/site identification and selection. This network has expanded significantly offering connectivity to more than 100,000 investigators across all continents barring one (sadly, Antarctica offers no clinical sites yet!).
Interestingly, working on clinical trials in various countries enabled us to develop channels with local service providers, freelancing teams (e.g. clinical trial monitors), regulatory specialists, and subject matter specialists, all local experts in their respective work areas.
Through these channels, Credevo is helping biopharma companies in various areas. Clients can easily execute projects in the following areas in any region with the help of local teams that Credevo builds for their project utilizing CROs (local, regional, international ones), freelance monitors, project managers, and experts.
1. Clinical trial operation
2. Site management
3. Regulatory support (including product registration)
4. Bioequivalence projects (China)
5. Pharmacovigilance
Besides this, of course, there is our core expertise that continues to help clinical teams.
6. Clinical trial feasibility, site identification, and site selection.
However, there is one area that has been appreciated a lot by clients, not only small-medium biopharma but also clinical research service providers.
Strategic consultancy & market intelligence support
An extensive network of clinical development service providers, sites, and investigators, as well as knowledge of local challenges and benefits, has helped clients in
- Development of their clinical development strategy
- Selection of best regions to place clinical programs
- Development of teams, and
- Understanding market conditions, hidden factors, and their utilization for growth.
One of our clients has saved millions of dollars utilizing strategic support from Credevo.
Understand and explore more
It will help a lot more to understand how this could be useful to your specific situation.
If you’re a clinical development-oriented bio-pharma, medical device, or biotech company; or, one that supports them in clinical development, there are potential benefits that you can draw from Credevo’s strategic support.
Since, each situation might vary a lot, it’s hard to cover them in this article.
But, I invite you to discuss with us. Contact Credevo or just send us a message through social media or other means (links are given below). Let’s get in touch and understand your needs and challenges.
We promise, we are very good listeners. 🙂
Kshitij
Contact Credevo team via Credevo.com
Explore Credevo’s works and free knowledge support through blogs
Contact or follow Credevo on social media: LinkedIn, Twitter, Facebook
Contact or follow Kshitij on LinkedIn