What You Need to Know to Register Nutraceutical, Dietary Or Food Supplement Products In Europe – Part I

Nutraceuticals, dietary supplements, or food supplements, also known as “Health Supplements,” have been used for years to maintain, enhance, and improve the healthy function of the human body. They may contain a combination of vitamins, minerals, amino acids, fatty acids, enzymes, probiotics, and other bioactive substances. Each country differs in its regulatory requirements for registration and marketing approval for such products. The European Union (EU) classifies these products as Food Supplements and regulates them as food or novel food.

The European Commission has established harmonized legislation that regulates the vitamins and minerals, and the substances used as their sources. Substances, excluding vitamins and minerals, intended for use in food supplements and lacking a history of safe use in the EU before 1997, classify as ‘Novel Food.’
In this blog, we will explore what Novel Food is and what the legislative requirements for obtaining marketing approval for such products are. On 1st January 2018, the EU introduced new regulations to obtain approval for Novel Food.
- The EU regulatory body, for the first time, introduced a centralized and more efficient authorization procedure for Novel Food products, with the EC managing them.
- The new process made it a lot easier and transparent for nutraceutical companies, to get a formal opinion from one regulatory body. It can be used across the European states and it can greatly expedite the process and reach the market.
- European Food Safety Authority (EFSA) plays a key role in this evaluation process and provides a safety assessment of the product.
What is Novel Food?
European Union defines “Novel Food” as food that had not been consumed to a significant degree by humans in the EU before 15 May 1997, when the first regulation on Novel Food came into force.
Novel Food is
- newly developed, innovative food,
- food produced using new technologies and production processes,
- as well as food which is or has been traditionally eaten outside of the EU
Traditional Food from a third country is the product where the history of safe food use in a third country has been demonstrated.
- People in at least one-third of the country have been consuming those foods for at least 25 years as part of the customary diet of a significant number of people.
- The history of safe food use should not include non-food uses or uses not related to normal diets.
The extended definition of Novel Food, through this regulatory recast, allows nutraceutical companies to market innovative products and traditional items from non-European countries to Europe. This is done while ensuring a high level of food safety for European consumers.
How does the sponsor determine the Novel Food status of a product?
- Sponsor/ food business operators should ascertain whether the food that they intend to place on the market falls within the scope of this regulation.
- When they are unsure about the scope of this regulation, sponsor/food business operators can contact the member state where they first intend to place the Novel Food.
- Sponsor/food business operators should provide the necessary information to the member state to enable them to determine whether or not a food falls within the scope of this regulation.
- To determine whether or not a food falls within the scope of this regulation, member states may consult the other member states and the Commission.
What is the current Novel Food legislation in Europe?
In November 2015, the European Parliament and the Council agreed to adopt EU Regulation (EC) 2015/2283 replacing Regulation (EC) No 258/97 of the European Parliament and Regulation (EC) No 1852/2001 of the Council and Commission.
This regulatory revamp of the Novel Food sets out harmonized rules for placing the Novel Food on the EU market.
Listed below are the main features and improvements of the new regulation.
1. Expanded categories of Novel Food
It’s foods originating from
- plants,
- animals,
- microorganisms,
- cell cultures,
- minerals, etc.,
- specific categories of foods (insects, vitamins, minerals, food supplements, etc.),
- foods resulting from production processes and practices, and state-of-the-art technologies (e.g. nanomaterials, intentionally modified or new molecular structure,),
Foods that were not produced or used before 1997 may therefore be considered Novel Foods.
2. Established Union list of authorized Novel Foods
A list containing all authorized Novel Foods permitted to market in the EU.
For future Novel Food authorization, the Commission implementing regulations will add new Novel Food products to the Union list. Once a Novel Food is on the Union list, it automatically obtains authorization and can be placed in the European Union market.
3. Generic authorizations of Novel Foods
Under the new regulation, all authorizations (new and old) are generic as opposed to the applicant-specific.
Any sponsor or food business operator can place an authorized Novel Food on the European Union market. They must maintain the authorized conditions of use, labeling requirements, and specifications similar to the original product.
4. A simplified, centralized authorization procedure
With this reform, the European Commission introduced a centralized and online application submission system.
5. Centralised, safety evaluation of the Novel Foods
The European Food Safety Authority (EFSA) will carry out the risk and safety assessment. The European Commission consults EFSA on Novel Food applications and provides its opinion based on the EFSA’s evaluation.
6. Efficiency and transparency
Establishing deadlines for safety evaluation and the authorization procedure greatly improved it. This reduction in overall approval time occurred through these changes.
7. A faster and more structured notification
To facilitate the marketing of traditional foods from countries outside the EU, a new regulation introduced a simplified assessment procedure for foods new to the EU.
This primarily aimed at traditional foods from third countries based on a history of safe food use.
Evidence of a history of consumption in the third country establishes the safety of the traditional food. If neither the EU countries nor EFSA raises safety concerns, the food can be allowed in the EU market.
8. Promotion of innovation
Granting an individual five years of exclusivity based on newly developed scientific evidence and proprietary data. The regulatory authorities may grant individual authorization to an applicant for placing Novel Food on the market.
Underlying EU principles for the Novel Food review process
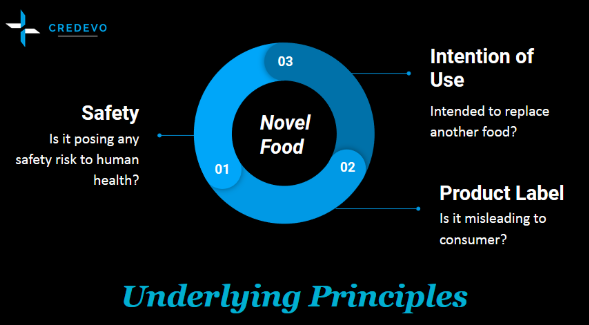
In the interest of the safety of the European consumers, the European Commission will only authorize and include a Novel Food in the Union list, if it fulfills certain conditions. These criteria for authorization of Novel Food in the Union list are
- Safety of the product – Based on the scientific evidence available, the food should not pose a safety risk to human health
- Product Label – The product label and its use should not mislead the consumer. The sponsor needs to ensure proper labeling of the product. Special attention needs to be given when the food is intended to replace another food and there is a significant change in the nutritional value.
- The intention of use – when the food is intended to replace another food, it should not differ from that food in such a way that its normal consumption would be nutritionally disadvantageous for the consumer.
Authorization procedure for Novel Food approval
Regulation (EU) 2015/2283 sets out the requirements for placing Novel Food products on the EU market by two means:
- New Novel Food
- Traditional food from a Non-EU country
Based on the product, the sponsor has to adapt to the registration process. Both pathways have their requirements. New Novel Food requires complete dossier submission whereas traditional food from non-EU countries can go through the notification process. For successful and timely submission and approval, the sponsor needs to ensure where his products fit, it can increase the chances of delay if queries are raised from EFSA or the Member States.
Are you planning to obtain regulatory approval for your Novel Food product in Europe?
Talk to us today.
You may have a query or need a plan for European registration of your food product.
We are here to help.
Note: “Ask Credevo Experts” will be charged @ $50 / inquiry. Any inquiry requiring more than 30 min of expert’s time will incur additional charges.
Check out the pathways documentation requirement, Novel food authorization process flow, and timelines associated with the Novel Food application in the next part of this series.
Stay tuned!
One thought on “What You Need to Know to Register Nutraceutical, Dietary Or Food Supplement Products In Europe – Part I”
Comments are closed.