DCGI Approval In India: A Complete Guide To Drug Regulations, Clinical Trials, & Patient Safety
The Drugs Controller General of India (DCGI) heads the Central Drugs Standard Control Organization (CDSCO), overseeing DCGI approval in India, regulation, and quality standards of drugs and clinical trials. As the national regulatory authority, DCGI ensures patient safety and compliance with the Drugs and Cosmetics Act, 1940.

DCGI in the Indian pharmaceutical and medical device industries is among the world’s most regulated, and rightly so. The products these businesses produce have a direct and significant impact on the public’s health. In India, the ultimate authority in this area is the Drug Controller General of India (DCGI), whose job is to ensure that all drugs, vaccines, and medical devices meet safe and effective national and international standards before they’re allowed to enter the marketplace.
For any life sciences company (biotech, pharmaceutical manufacturing industry) that operates in or sells to the burgeoning Indian pharmaceutical market, understanding the DCGI’s role, rules, and procedures is a matter of regulatory necessity. However, even more so, it’s a governance pathway to innovation and growth for the Indian and the global pharmaceutical sector.
In this article, we cover the role and importance of DCGI, approval timelines and fees, regulatory framework under NDCT Rules, stepwise approval pathways, ongoing processes, annual approval trends, global acceptance of DCGI studies, and the future outlook of India’s regulatory landscape.
1. Work, approval timeline, and fees
The work of the DCGI is a meticulous, multi-stage process designed to thoroughly vet a product before it is approved. The journey begins with the submission of an application through the online SUGAM portal, a centralized system for all regulatory submissions. The application and approval process for a new drug is governed primarily by the New Drugs and Clinical Trials (NDCT) Rules, 2019. This pathway involves a series of steps, each with its own timeline and associated fees. For a new drug, the application is submitted using a specific form (Form 44). The fees are substantial:
| Step/Activity | Fee (INR) |
|---|---|
| Pre-submission meeting | 500,000 |
| Post-submission meeting | 50,000 |
| Application for manufacturing a new drug/IND for a CT or BA/BE study | 5,000 per product |
| Reconsideration of application for Manufacturing New Drug/IND for CT or BA/BE study | 2,000 per product |
The approval timeline for a new drug can vary depending on various factors or conditions. While some stages can be completed within 90 days, full market approval usually takes 12–18 months.
This depends on the completeness and quality of the submitted data. It also varies if regulators request clarifications.
2. Rules and regulations
The regulatory framework for drugs and clinical trials in India has evolved significantly to improve the standard and quality. The cornerstone of the current system is the New Drugs and Clinical Trials (NDCT) Rules, 2019, which replaced the older, more complex system under the Drugs and Cosmetics Act, 1940, and its Schedule Y. These rules provide a clear, detailed, and streamlined framework for the entire approval process currently in place.
Key aspects of the NDCT Rules, 2019, include
- Clear definitions: It clearly defines what constitutes a “new drug,” including new chemical entities, new fixed-dose combinations, and drugs with new therapeutic indications.
- Subject expert committees (SECs): The rules formalize the role of Subject Expert Committees, which are composed of external experts who provide recommendations on applications. These committees are instrumental in the scientific evaluation of a product’s safety and efficacy. Recent guidelines have standardized the operation of these committees to enhance transparency and consistency.
- Specific timelines: The rules establish specific timelines for the review and approval process, which helps to increase predictability and reduce delays for applicants.
3. Pathway of approval
Getting a new drug approved isn’t just a single step; it’s a carefully structured journey. From early lab research to clinical trials and regulatory reviews, each stage plays a vital role in proving the drug’s safety, effectiveness, and quality. It’s a process designed to protect patients while ensuring that promising treatments reach those who need them most.
- Application submission
- Scrutiny and review
- SEC review
- DCGI decision
- Grant of approval
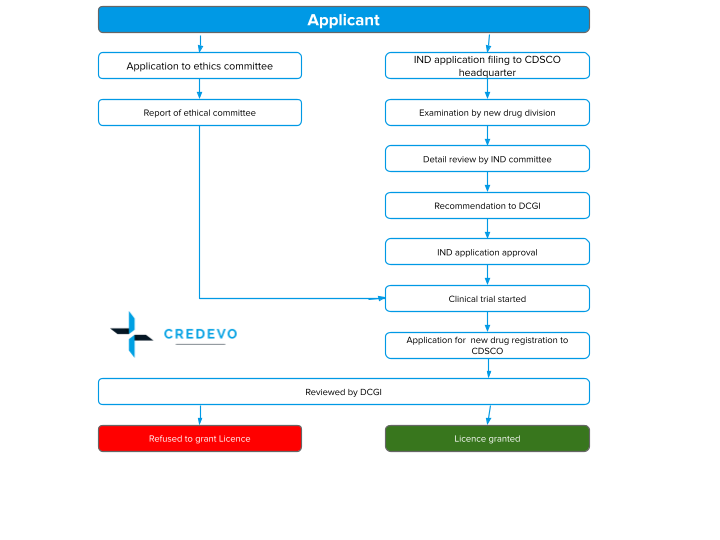
4. Pathway of work
Getting DCGI approval isn’t a one-and-done task. It’s an ongoing cycle where regulatory teams prepare detailed documentation and submit it with precision. They must stay closely engaged to address queries, provide clarifications, and keep everything on track. It’s a rhythm of planning, responding, and refining that demands both strategy and persistence.
Do you want to understand how early engagement of stakeholders can transform clinical trials? Click here
5. Number of studies approved every year
India’s clinical research portfolio has grown significantly over the past 20 years. The DCGI has played a key and important role in this huge transformation. Between 2004 and 2018, it approved 453 new drugs. From 2008 to 2022, the country conducted 220 first-in-human Phase 1 trials, showing increasing confidence and ability in early-stage research. Additionally, these approvals cover a wide range of therapeutic areas, with notable activity in oncology, neurology, infectious diseases, and gastrointestinal/metabolic treatments. This progress reflects how India is establishing a more important role in global drug development.
During the COVID-19 pandemic, over 150 meetings of the Subject Expert Committee (SEC) were convened to evaluate drugs and vaccines.
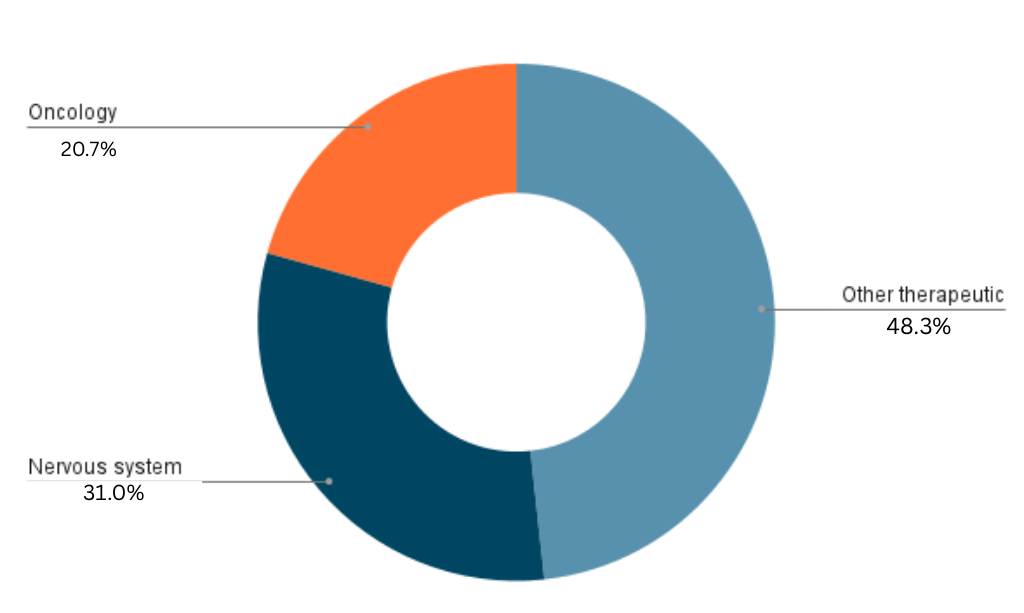
The chart shows that Other Therapeutic Areas account for the largest portion of approvals at 28%, followed by the Nervous System at 18%. The smallest portion of approvals is Oncology at 12%.
6. Acceptance of DCGI-approved study worldwide
Indian clinical research has come a long way. Not too long ago, data from DCGI-approved studies struggled to gain traction internationally. But things are changing fast. With DCGI and CDSCO now aligning their regulations with global benchmarks like those set by the WHO, the credibility of Indian trial data is steadily rising.
A major turning point has been the government’s move to waive local trial requirements for drugs already approved in trusted countries like the US, UK, Japan, Australia, and Canada.
One DCGI-approved study alone might not unlock approvals in places like the US or EU. However, the high-quality, GCP-compliant trials conducted in India are increasingly seen as valuable pieces of the global evidence puzzle. They help speed up access to life-saving treatments and put India firmly on the map in international drug development.
7. Future scope of DCGI approval in India
A focus on transparency, efficiency, and global alignment marks the future of the DCGI and the Indian regulatory landscape. The recent introduction of guidelines for Subject Expert Committees and revised guidelines for biologics are prime examples of this evolution. Future initiatives include:

8. Conclusion
The path to approval is a partnership between innovators and the regulator, united by the common goal of bringing life-saving products to those who need them most. The role of the Drugs Controller General of India is pivotal to the country’s healthcare ecosystem. Far from being a bureaucratic hurdle, the DCGI acts as a critical enabler of safe, effective, and accessible healthcare. The regulatory journey, while rigorous, is a testament to the country’s commitment to public health. With the implementation of the NDCT Rules, 2019, and a clear vision for the future, the DCGI is not only safeguarding India’s population but also positioning the country as a major player in global pharmaceutical research and development.
Do you want expert guidance to navigate DCGI approval in India and ensure your clinical trials meet all regulatory and patient safety requirements?
If you’re looking to engage with the DCGI to accelerate approvals, ensure compliance, or strengthen your global development strategy, feel free to contact our team using the form below. We’ll be glad to support you.