Medical Device Regulatory And Approval Process In Malaysia

There is a growing demand for medical devices in Malaysia, and overseas manufacturers are showing interest in registering and marketing their medical devices in Malaysia. The Medical Device Authority (MDA), the regulatory body for the medical device industry in Malaysia, projects that the market will grow at a 16.1% annual rate to reach US$1.4 billion. The pharmaceutical market is likewise sizable, with an estimated US$3.6 billion market with a CAGR of 9.5% growth rate.

Malaysia has been working to increase public access to healthcare in recent years as middle-class income growth fuels the demand for more precise diagnostic tools and medical gadgets to identify and treat chronic diseases. In Malaysia, imported and expensive products make up the majority of the market for medical equipment.
Registration is now necessary for any medical equipment, although the Malaysian government offered an optional registration process for medical device enterprises and equipment in the past.
The regulatory body for medical device registration in Malaysia
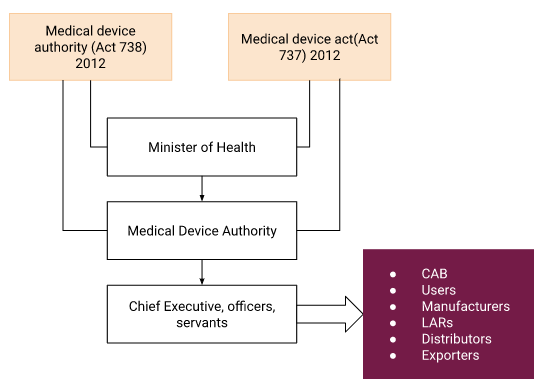
- The Medical Device Authority (MDA) is a statutory body under the Ministry of Health Malaysia. The MDA was created following the Medical Device Authority Act 2012 (Act 738) to control, regulate, and enforce the Medical Device Act 2012’s provisions regarding medical devices, their industry, and related activities (Act 737).
- Medical devices must undergo a registration process before selling in Malaysia. All overseas producers must register their medical devices with the MDA as of July 1, 2016, per the Medical Device Act of Malaysia.
Malaysia Medical Device Regulatory System flow
Medical device classification in Malaysia
To register your medical device in Malaysia, first, you need to determine the medical device’s class according to Appendix I of the Medical Device Regulations 2012, as published by the Medical Device Authority (MDA). Similar to the European Medical Devices Directive (MDD) 93/42/EEC, the MDA categorization regulations.
- The device’s classification is determined by; the risk it poses when in use (The risk to patients, users, and other persons).
- A device’s potential risk is determined by:
- Its intended objective
- Risk management strategies
- Manufacturing, and use
- Intended clientele (s)
- Technology’s way of functioning
Medical Devices Classification in Malaysia
| Class | Risk |
|---|---|
| Class A | Low danger (blood pressure cuffs, scissors, etc.) |
| Class B | Low to moderate risk |
| Class C | Moderate to high-risk |
| Class D | Highest risk (implantable devices or devices that materially alter human physiology). |
Rules for IVD Classification
Separately from other medical devices, IVDs fall under the following categories ranked from lowest to highest risk: Class A, Class B, Class C, and Class D.
The risk for IVD is determined by;
- The intended application
- The intended user’s level of competence
- The significance of the information obtained from the diagnostic implications of the test results
Are you looking for Pharmaceuticals regulatory in Malaysia, Click here to learn.
Requirements for Medical Device Registration
The following documents are necessary for the registration form for medical devices:
- Information in general about the medical device
- Information about the medical device’s maker
- The assemblage of medical equipment
- Template for a Common Submission Dossier (CSDT)
- History of post-market vigilance
- Statement of compliance
- Certification for the registration of medical devices
Approval process
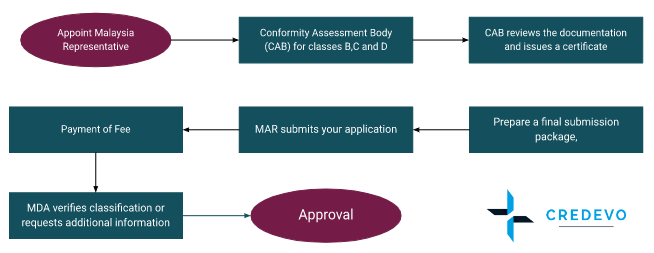
Malaysia representative
You may need a Malaysia Authorized Representative to handle your medical device registration with the MDA if you don’t have a local presence in Malaysia. Each device may only have one Authorized Representative appointed by the manufacturer.
Throughout the registration procedure and following registration approval,
- The Authorized Representative will represent the foreign producer as its local agent (license holder).
- Additionally, the AR is in charge of required post-market surveillance tasks.
- The AR may be your Malaysian subsidiary office, a Malaysian distributor, or a neutral third party.
- The benefit of using a third-party independent AR is that the distributors don’t have access to confidential technical information.
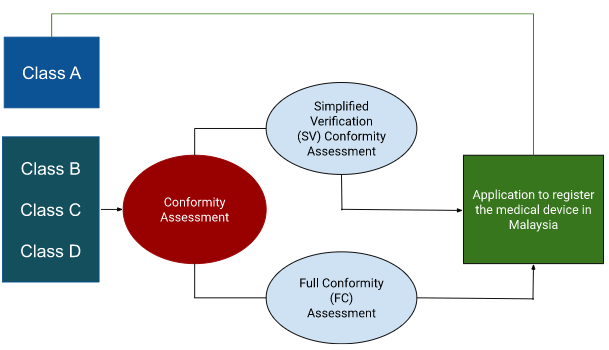
Conformity Assessment Body (CAB) for classes B, C, and D
Conformity Assessment Bodies (CAB) are MDA-certified third parties in Malaysia. CAB offers two medical device conformity assessment services.
- Assessment of simplified verification (SV)
- Full confirmation (FC) evaluation
Local and international manufacturers who have previously received approval for the registration of medical devices from at least one of the five GHTF nations (the European Union, the USA, Japan, Australia, and Canada) are eligible for the much simpler SV assessment pathway.
Everyone else must undergo the laborious FC assessment, where the documentation requirements are similar to those for obtaining the CE certification.
Conformity evaluation is a systematic and continuous review of the available data and methods to guarantee;
- The efficacy
- Reliability
- Benefits, and
- Hazards of medical devices.
Before being used, all class B, C, and D medical devices must pass a conformity assessment. Class A medical devices are excluded from conformity evaluation by a CAB before submission with the MDA due to their minimal risk potential.
Based on technical data provided by a foreign manufacturer, the AR creates a Common Submission Dossier Template (CSDT) and uploads it electronically to the Malaysian Medical Device Authority (MDA).
Review the documentation and issue a certificate by CAB.
The applicant shall prepare a final submission package for each device that includes the CSDT, the CAB certificate, and the MDA application forms.
Submission of application
Malaysia Authorized Representative submits your application electronically via the MDA Medical Device Centralized Online SystrequestC@St).
Documents shall be in the English language. Devices intended for domestic usage must include Bahasa Malaysian labels and IFU.
Payment of fee
As follows, by device categorization, the cost of registration applications and assessments varies as follows:
| Device Classification | Application fee | Processing fee |
| Class A | MR 100 | n/a |
| Class B | MR 250 | MR 1000 |
| Class C | MR 500 | MR 2000 |
| Class D | MR 750 | MR 3000 |
| Combination devices | MR 750 | MR 5000 |
MDA charges these costs (excluding the charge for CAB review).
MDA verifies classification and/or requests additional information
The MDA examines categorization. If there are any flows in the application, you will have to provide more information or proof; your application gets rejected if you can not meet the demands.
Issue of authorization letters
A Certificate and Registration Number are both given upon approval. The holder needs to renew registrations and CAB certificates every five years.
Your Malaysia Authorized Representative must issue authorization letters permitting your device’s importer or distributor to carry out those tasks.
The time frame for the medical device approval process
Medical Devices and IVDs (official timeline, excluding timeline for CAB review)
| Class | Timeframe |
|---|---|
| Class A | 45 working days |
| Class B | 100 working days |
| Class C | 180 working days |
| Class D | 220 working days |
License validity and transfer of license
- The permanence of the license is 5 years
- Transfer of license – A procedure known as “Change of Ownership” allows for the transfer of licenses.
Need Support To Register Your Medical Device In Malaysia?
Do you have any questions or need regulatory support to register your medical device in Malaysia? Provide brief details in the below form to connect with us