Brunei Generic Drug Registration Process

The Asia-Pacific region is emerging as a new destination for generic drugs. The Asia-pacific market is forecasted to grow from USD 75 billion in 2020 to USD 112 billion by 2025 at a CAGR of 8.34%. The growth of the generic drugs market is accelerated by many factors such as the aging population, increasing pharmaceutical spending towards generics, and the impending expiry of patented drugs.

The attractive pharmaceuticals market growth in ASEAN countries like Vietnam, Singapore, Indonesia, Malaysia, Philippines, Thailand, Brunei Darussalam, Laos, Myanmar, and Cambodia are becoming the market of interest for many pharmaceutical companies.
Brunei Darussalam’s pharmaceutical market is anticipated to grow owing to the increased non-communicable disease burden and the implementation of action plans by Brunei’s regulatory authority to address the prevention and control of diseases.
The regulatory environment among all the ASEAN countries is quite similar but still, the regulatory requirements and the process of registration significantly differ. Hence, understanding the process of registration & regulatory requirements can be advantageous in order to register a medicinal product and enter into the market.
Let’s understand the regulations for generic drug registration in Brunei and the registration process.
Who can apply for registration?
In order to enter the Brunei Darussalam market, a medicinal product must be registered and the manufacturer’s local representative or its appointed agent entities are responsible for applying for the registration of medicinal products:
Regulatory for generic drugs in Brunei
A generic product applies to any medicinal product similar to a currently registered product in Brunei Darussalam.
- The Ministry of Health (MOH), through the Department of Pharmaceutical Services (DPS), regulates the registration of all medicinal products for human use in Brunei.
- The Brunei Darussalam Medicines Control Authority (DMCA) through the Department of Pharmaceutical Services (DPS) is responsible for making decisions concerning any matter related to registration and the issuance of certificates and licenses to grant, renew, vary, suspend and revoke medicinal products under Medicines Order, 2007.
The registration process for generic drugs in Brunei
After understanding the complete Brunei regulatory for registering the generic drugs in Brunei, one can start the process of registration by collecting all the documents and certifications required for submission for generic drug registration.
Medicinal products registered with the regulatory authorities in some other countries facilitate the registration process in Brunei.
The registration process and regulatory requirements for generic products in Brunei can be summarized into simple steps as below
- Pre-submission preparation
- Submission of the application dossier
- Evaluation of the application
- BDMCA Approval
Need support for your drug registration in Brunei Darussalam?
Credevo offers expertise in drug product registration, clinical trial regulations, and many more services in Brunei. Check them out now!
Pre-submission Preparation
Application dossiers for registration of medicinal products must comply with the ASEAN Common Technical Dossier (ACTD) and ASEAN Common Technical Requirements (ACTR). All documentation must be done in either English or Malay.
Generic products do not require non-clinical documents and clinical documents. However, DRU may request clinical study reports if necessary. The application dossier for generic products includes only Part I and Part II
Bioequivalence studies
The bioequivalence studies are necessary to register most generic products in Brunei. This is to ensure the therapeutic equivalency with the innovator’s product and clinically interchangeability.
If biowaiver is granted by a certain regulatory authority, then a justification can be provided for consideration to the DRU for biowaiver in Brunei.
Applicants are required to be familiar with the ASEAN Guideline for the Conduct of Bioequivalence Studies for registration.
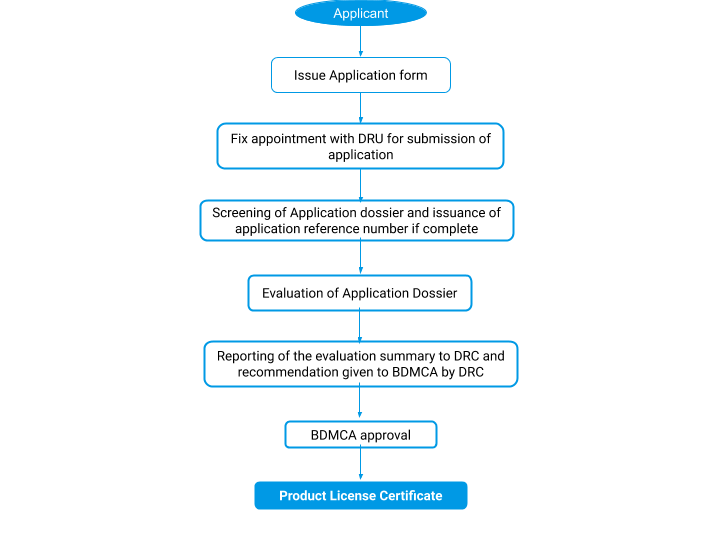
Submission of the Application
Application forms are issued from the DPS for the registration of medicinal products. The application dossier is screened for completeness and an application reference number will be assigned if the submitted dossier is in proper order.
Applicants will be informed in case of incompleteness and sent back to the applicant.
Evaluation of the Application
The evaluation of the application dossier for the registration of medicinal products follows a proper sequence.
- The DRU may request additional information from the applicant during the evaluation of the application for registration.
- After verification of the application dossier, the evaluation report summary is forwarded to the Drug Registration Committee (DRC) for a recommendation.
- The final decision is given by BDMCA upon recommendation from the Drug Registration Committee (DRC).
BDMCA Approval
The decision of the Brunei DMCA in writing is given to the applicant whether the application has been approved or rejected. A Product Licence Certificate (PLC), and Product Licence Number (PLN) will be issued by the Brunei DMCA for approved medicinal products for registration in Brunei Darussalam. A product license certificate will have a validity of 5 years.
Approval process flow
Registration Timelines
The entire process right from making an appointment with Brunei DRC to issue of certification to the sponsor takes about 286 days approximately.
Processing fees for Registration
The fee depends on the type of application
- Fee for issuance of the application reference number
- Fee for issuance of the Product Licence Certificate for 5 years validity
- Fee for renewal
Post-Approval Conditions
Once the sponsor receives the approval for the product, the sponsor needs to follow some conditions such as the registered product should be identical in all aspects to that approved and no change can be made without prior approval by DMCA.
The product license holder must provide information related to the medicinal product as and when required by DMCA. The product license number must be labeled within 8 months from the date of BDMCA meeting.
Import License
According to the Medicines Order, 2007 before the manufacture, supply, sell, or import of medicinal products into Brunei Darussalam, the product must be registered and the responsible person must have an appropriate license.
The Import Licence will authorize the applicant to import, store, and wholesale or supply registered medicinal products. The applicant has to apply for an import license before 3 months of importation.
The validity of an Import Licence is one year and renewal can be applied 3 months before expiry.
Do you need support or have queries on drug registration requirements?
Credevo offers a wide range of drug development and regulatory services in Brunei. Choose one of the following options to connect with us.
Get the report on the generic drug registration process in Brunei.
Note: This report will be charged @ $359.
Do you have a query? Just ask experts at Credevo.
Note: “Ask Credevo Expert” will be charged @ $50 / inquiry. Any inquiry requiring more than 30 min of expert’s time will incur additional charges.
Looking for a quotation? Just provide relevant info and we will send you the details.
References
- http://www.moh.gov.bn/SitePages/Registration%20Medical%20Product.aspx
- http://www.moh.gov.bn/Shared%20Documents/Traditional%20Medicines/Importing%20Medicines/Registration/Guide%20to%20application%20for%20registration%20of%20medicinal%20products%20-%204th%20editi.pdf#search=generic%20drugs%20registration%20process
- http://www.moh.gov.bn/SitePages/Importation%20of%20Medicines_2020.aspx
- https://bdntr.mofe.gov.bn/Import%20Certification/MOH%20-%20Pharmaceutical/Guideline%20on%20Application%20for%20an%20Import%20Licence%20and%20Wholesaler%E2%80%99s%20Licence%20for%20Medicinal%20
- https://www.marketdataforecast.com/market-reports/asia-pacific-generic-drugs-market
- https://www.fitchsolutions.com/topic/brunei-darussalam
- https://www.ijrpc.com/files/13-01-15/15-520.pdf
- https://www.makrocare.com/WhitePapers/se-asian-regulatory-environment-p2.pdf