Clinical Trial Designs: Basket, Umbrella & Platform Trial Designs – Part II

The US FDA recommends efficient clinical trial design strategies such as basket designs, and umbrella designs to expedite the development of oncology drugs and biologics. FDA also provides guidance and advice to sponsors of drugs and biologics for cancer treatment regarding the design and conduct of clinical trials, other than first-in-human (FIH) trials.

In contrast to traditional trial designs, which tests a single drug in a single disease population in one clinical trial, In contrast to traditional trial designs, the master protocols use a single infrastructure, trial design, and protocol to simultaneously evaluate multiple drugs and/or disease populations in multiple substudies, which allows efficient and accelerated drug development.
In the previous article, we have seen the RCT and adaptive clinical trial designs. In this article, we will discuss the most efficient clinical trial designs like basket (bucket), umbrella & platform trial designs.
Before going into the details of various trial designs, let’s understand what is a master protocol?
What is the master protocol?
A master protocol is a study protocol design with multiple substudies, which may have different objectives and involves coordinated efforts to evaluate one or more investigational drugs in one or more disease subtypes within the overall trial structure.
The sponsor may design the master protocol with either a fixed or adaptive design intent to modify the protocol to incorporate or terminate individual substudies within the master protocol.
These master protocols offer unique and flexible designs and incorporate therapies with different mechanisms of action, biomarker development, and genetic subtyping.
What are the novel master trial designs?
Master protocols, classified as basket trials, umbrella trials, and platform trials are novel designs that investigate multiple hypotheses through concurrent sub-studies. For example, multiple treatments or populations or that allow adding/removing arms during the trial. These offer enhanced efficiency and a more ethical approach to trial evaluation.
These designs are widely used in oncology trials and are suitable for many other disease trials.
Despite the many advantages of these designs and the FDA recommendation for using such designs, many researchers tend to still use traditional designs due to a lack of knowledge or a well-established strategy in clinical drug development.
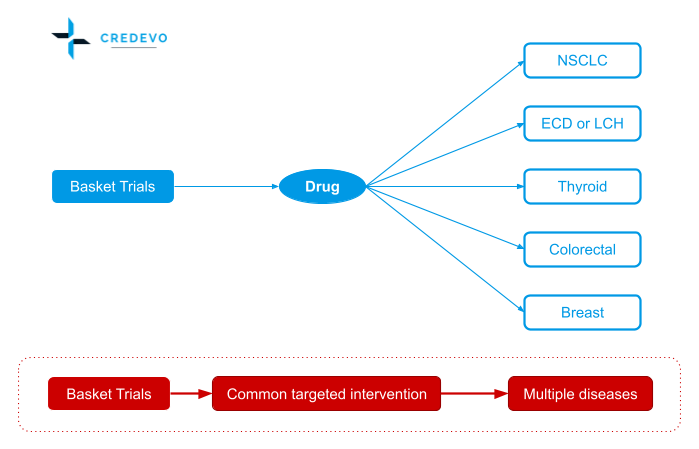
Basket trial designs (Bucket trials)
Basket studies are also called bucket studies. A type of clinical trial design that tests how well a new drug or other substance works in patients who have different types of cancer that all have the same mutation or biomarker.
Traditionally clinical trials would focus on the treatment of cancers for some specific genetic mutation or at certain locations such as lung, breast, etc. A more recent development for oncology clinical trials, such as a basket study, includes patients with a particular genetic mutation in common regardless of the site of origin of cancer in the body.
For example, patients recruited in the basket study have the same genetic mutation, but cancers locate in different regions such as lung, liver, prostate, etc.
Basket trials can be relatively simple in design to include specific treatment arms or ‘baskets’ for cancers of different origins or locations. The design can also become more complex with the baskets including more than one genetic mutation across multiple cancer locations. Or design to evaluate multiple drugs across a selected number of genetic mutations and cancer locations.
Advantages of basket trials
- Basket studies can determine if a drug targeting genetic mutation at a particular site may be effective in treating that same genetic mutation found in cancer located in another site of the body.
- In terms of origin or location of cancer,.these designs help to evaluate if a drug would be a good candidate for larger trials with a more specific target,
- The US FDA considers a basket study to be adequate evidence for approval and recommends researchers utilize such trial designs.
- For already approved drugs, treating cancer with a specific genetic mutation at one location, a basket study can be useful to see if the efficacy translates to cancer at other locations in the body. As only one genetic assay is required for screening patients for study enrollment.
- Exposure in multiple contexts can provide an additional understanding of the mechanism of sensitivity and resistance.
Drawbacks
- Some baskets may have small sample sizes if the mutation is rare.
- Without a comparative arm, the researcher can’t distinguish predictive from prognostic.
- Single-arm sub-studies generally require a tumor response rate endpoint.
- Challenging to define historical controls across diseases.
Basket trial experiences
For example, in a basket study involving patients with BRAF genetic mutation, researchers found that vemurafenib was also effective in treating a rare blood cancer known as Erdheim-Chester Disease (ECD). Patients have BRAF V600 genetic mutation. This basket study helped lead to the eventual FDA approval for vemurafenib to treat BRAF ECD in 2017.
Basket design patterns
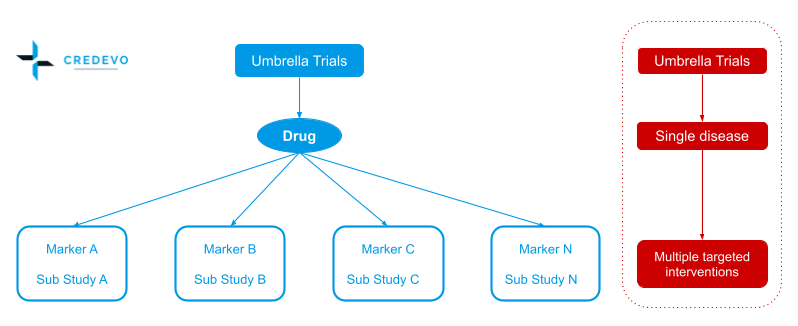
Umbrella trial designs
In umbrella trials, patients are enrolled with one cancer type but with different genetic changes within each tumor and consist of many small sub-trials to test multiple drugs simultaneously in one large trial.
- Patients receive different targeting treatments matched to their genetic aberration.
- The term “umbrella” refers to the separation of one alleged cancer into many sub-cancers depending on their molecular features.
- Sub-studies may be single-arm, phase II, or phase II/III trials that are randomized and compared to placebo or standard therapy.
- There is also a “default arm” which assigns patients without a specific marker to receive the standard treatment.
- Umbrella trials are often single-arm or randomized sub-studies that are confirmatory.
Umbrella trial experiences
In Lung-MAP patients treated previously with advanced squamous cell lung cancer, initially three parallel randomized phases II/III sub-trials for targeted therapy vs. SOC (docetaxel). The goal was to screen 500-1,000 patients per year and Contain the 4th cohort: a non-match study for patients not eligible for target cohorts.
Advantages
- The umbrella trials in oncology offer a new trial design aiming to test different types of treatments innovatively and effectively.
- Minimizes the risk for participants, maximize expected benefits and expected benefits are maximized, and
- The possible benefits to participants and society outweigh or are proportional to the risks associated with participation in the study.
Drawbacks of umbrella trials
- Require larger size, particularly in randomized sub-trials.
- Longer duration.
- Difficulty enrolling rare molecular subtypes of a single tumor type.
- Susceptibility to changes in the “treatment landscape” during the trial.
Umbrella trial design patterns
Super umbrella trials
The combination of the bucket trials and the umbrella trials creates the “Super Umbrella Trials”
Platform trials
Platform trials referred to as Multi-Arm, Multi-Stage (MAMS) design trials evaluate several interventions against a common control group and can be perpetual. These designs help to further accept additions or exclusions of new therapies or patient populations during the clinical trial.
- Basket and umbrella trials can also include platform trials if they permit the addition or exclusion of new treatments during the trial.
- In a platform trial, interim analyses evaluate the efficacy or futility of each targeted therapy and use their results to add new ones or exclude certain targeted therapies.
Advantages
- Platform trials permit relatively flexible addition or exclusion of treatment methods or patient populations, enabling an efficient transition to a confirmatory clinical trial.
- Do not require a new trial infrastructure for every treatment under investigation, and It allows the sharing control group means require less recruitment of patients.
Drawbacks
- Challenges of the platform trial include its large-scale, long-term nature, high costs of managing and executing the trial and
- Needs to build organizations or frameworks that can operate these trials perpetually.
Other than the above-mentioned trials there are also some more trial designs like
Seamless design, where researchers combine the learning stage of Phase II trials and the confirmatory stage of Phase III trials and
Internal pilot design where patients are few, as in the case of rare diseases, allocating them to a pilot study rather than the definitive study could be seen as a wasteful approach.
However, such trials are planned or designed during clinical strategy development to make an efficient design to overcome the hurdles that may come across while conducting trials and attaining valid clinical trial data.
Looking to prepare/improve protocol design?
Building sound clinical development strategies and selecting the right design is immensely helpful for the successful and accelerated completion of your clinical trial.
For existing protocol designs, we recommend getting help from experts for improvisation to help in achieving targets.
Note: “Ask Credevo Expert” will be charged @ $50 / inquiry. Any inquiry requiring more than 30 min of the expert’s time will incur additional charges.
However, if protocol design is still being prepared, it’s a great opportunity to optimize it from a holistic perspective.
References
- https://med.stanford.edu/content/dam/sm/cisd/symposium-May2017-slides/EricPolley-May2017-symposium.pdf
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5380524/
- https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-019-3664-1
- https://med.stanford.edu/content/dam/sm/cisd/symposium-May2017-slides/EricPolley-May2017-symposium.pdf
- https://www.pharmacytimes.com/contributor/ryan-chandanais-ms-cpht/2018/07/basket-studies-an-innovative-approach-for-oncology-trials