Poland – Clinical Trial Advantages And Regulatory Process

Poland is regarded as one of the largest clinical trials markets in CEE/CIS. In a report in 2010, PriceWaterhouseCoopers (PwC) pointed out that with 469 new clinical trial registrations in 2009, Poland accounted for c. 2.5%-3.0% of the world market by registration volumes.

Current status of clinical research in Poland
- The status of clinical research has degraded over the years in Poland. In a subsequent report in 2015, the same agency (PwC) indicated that Poland’s clinical trials market has been stagnant in recent years.
- Between 2012 and 2014, there was a drop in the number of new clinical trial registrations, which can be attributed to a number of both local and global factors. One of these factors was reported as lengthy administrative procedures and a large number of formal requirements in Poland.
Despite these requirements, Poland carries certain advantages in clinical trial conducts that are hard to ignore.
Are you looking for regulatory support in various stages of drug development? write to us at helpdesk@credevo.com or provide your query in the form below
Why Poland still is a good clinical trial site?
Large population of patients
- Poland has a relatively large population in comparison with neighboring countries.
- Moreover, there seems to be a greater motivation for patients in Poland to become involved in clinical trials, compared to mature Western European markets.
- This is because patients participating in trials in Poland usually have access to medical treatment of a higher level than is the case of standard care.
Specialized medical centers
- There are a considerable number of specialized medical centers clustered around main cities (e.g. Warszawa, Wrocław, Kraków, Poznań, Szczecin, Gdańsk, Lublin, Bydgoszcz, Rzeszów).
- These centers have well-qualified specialists and provide access to patients in all therapeutic areas, particularly oncology, rheumatology, cardiology, and pediatrics.
- As per the report (referenced from the Polish Central Statistical Office GUS report ) in 2014 indicates the number of hospitals and healthcare facilities as more than 800 hospitals and 16,600 outpatient facilities.
Highly interested, qualified and motivated investigators and site staff
Polish investigators are generally highly motivated to conduct clinical trials, as they can see the benefits in the work they do more than their colleagues in Western countries. Clinical trials are attractive to investigators because of
- Possibility to test new treatment standards,
- Exchange information with foreign experts,
- Financial benefits, and
- Opportunity to have co-authored publications in respected branch magazines.
General rich experience of over 20 years is well reflected in the quality of clinical research data from Poland provided by investigators and site staff.
It is worth noting that no Polish investigator is present on the FDA’s list of disqualified investigators.
Relatively low costs
- Poland holds a strategic advantage in having a much lower cost of conducting clinical trials (nearly 30% less) than in the U.S.
- This is due to a high rate of patient recruitment and excellent quality of data, leading to a reduced number of rejected clinical trial recordings and time-efficient proceedings.
Top challenges with clinical trials in Poland
However, there are some key challenges for the Polish clinical trial market, which concerns some companies. These challenges include
- A regulatory process requiring the signing of clinical trial agreements first
- Administrative procedures regarding the signing of a clinical trial agreement at different sites may differ with the type of the site.
To overcome these challenges and utilize the above-mentioned advantages, it helps to understand the clinical trial regulatory process and expected timelines.
Clinical trial regulations & processes in Poland
Like many other countries, Poland has 3 different approvals required generally.
- Regulatory authority (RA)
- Ethics Committee (EC), and
- Import license
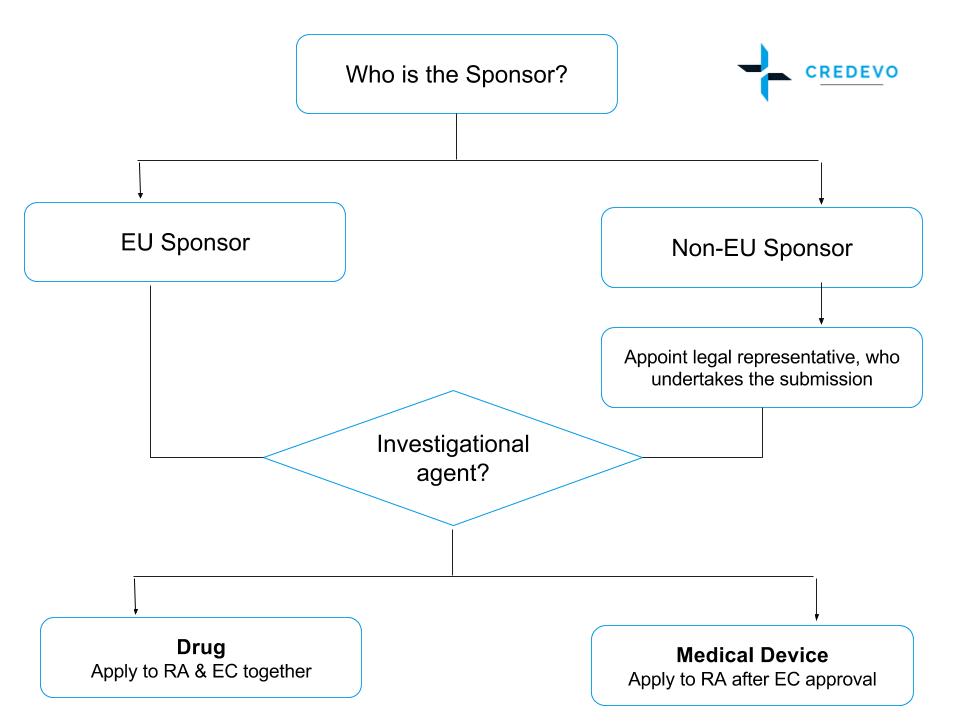
Figure 1: Clinical Trial Regulatory Process in Poland
Submissions to RA & EC differ as per the type of sponsor and investigational agent.
- The Clinical Trial Application (CTA) should be submitted to the RA by the clinical trial sponsor or its authorized representative.
- Before the CTA is filed to the RA and EC, all non-EU sponsors must appoint a so-called ‘Legal Representative’ that has to be legally established in the European Union.
- Application for drug studies can be made to RA and EC in parallel, however, the CTA for medical devices must be made to the RA after EC approval has been obtained.
Regulatory Agency (RA)
The sponsor must apply for authorization to conduct a clinical trial on an investigational agent (IMP) to the competent authority in Poland, which is the Office for Registration of Medicinal Products, Medical Devices, and Biocidal Products, one department of which is responsible for GCP assessment and maintains the Central Register of Clinical Trials.
When applying to RA, the sponsor or their representative must submit the following documents.
Documents
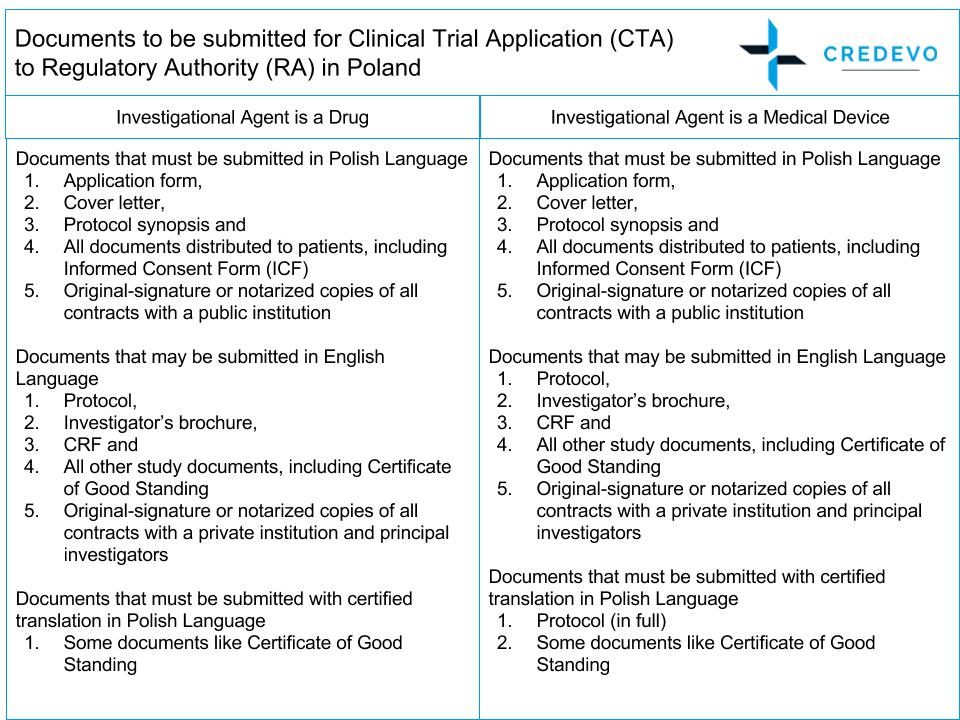
Figure 2: Documents to be submitted for the clinical trial application to RA in Poland
Some additional but important notes.
- The CTA approval process is 60 days long.
- RA requires that the contracts for all participating sites in Poland must be signed and submitted to RA before the CTA submission (medical device studies) or between the CTA submission and the end of the 60-day period of the CTA approval process (drug studies).
- To expedite the process in large studies, the initial request may be made for a reduced number of sites.
- Following the initial RA approval and when the site-specific documents, including signed contracts for the remaining sites, are ready for submission, the request may be amended to add these clinical sites.
- The review and approval of additional sites by the RA can take up to 35 days.
Ethics Committee (EC)
Salient points for submission of documents and process of EC approvals for clinical trials in Poland
- From all Principal Investigators participating in the study in Poland, a Country Coordinator must be chosen and his/her local EC becomes the Central EC (CEC) responsible for review and approval of the whole study.
- The CEC issues the single opinion on the clinical trial and directly informs about the study of the local ECs of all participating Principal Investigators.
- The local EC then within 2 weeks is responsible for giving an opinion on the investigator and the site facilities.
- If the local ECs will give favorable opinions about the sites or will not respond during the mentioned above timelines, the study is considered approved for all sites submitted within the application.
- The law forbids payment to patients for taking part in a clinical trial, other than their traveling expenses.
- Loss of earnings might be included in the lump sum.
- Payment may be made to healthy volunteers taking part in bioavailability studies, or Phase I studies conducted in Poland. But, payment for “sick” volunteers is forbidden.
- The period of EC approval is generally 40-45 days in Poland.
IMP clinical trials
- If the study drug or the medical device will be imported from an EU country, there is no need for an import license.
- However, if the import will be done from a non-EU country, then an import license is required from the RA.
- The quantity of the study drug or medical device to be used in the study must be specified – as per standard practice, a surplus of 25 % may be included in the request to ensure the sufficient number of study supplies are imported to Poland.
Clinical trial sites & investigators in Poland
With high levels of subject recruitment, high data quality and committed investigators, and relatively low costs, Poland is an attractive location for clinical research.
These advantages, clear knowledge of regulatory requirements, and quick connectivity with sites and investigators in Poland can ensure a smooth, quick, and cost-effective execution of clinical trials.
Need Support for conducting your clinical trials in Poland or Have questions?
We’d love to help you conduct clinical trials in Poland.
Provide your details below.
References
- https://www.pwc.com/gx/en/pharma-life-sciences/assets/clinical-trials-in-poland-2010.pdf
- https://www.gcppl.org.pl/Portals/2/reports/Clinical-Trials-in-Poland_12-2015_FINAL.pdf
- http://cropoland.com/requirements.html
- https://www.cromsource.com/wp-content/uploads/2012/12/Clinical-Trials-in-Poland.pdf
- http://www.urpl.gov.pl
- http://www.nil.org.pl/
One thought on “Poland – Clinical Trial Advantages And Regulatory Process”
Comments are closed.