Early Site & Investigator Engagement In HR⁺/HER2⁻ Breast Cancer Trials: Foundations For Better Trial Design
Early site and investigator engagement are the foundations of effective clinical trial design, ensuring that protocols are both practical and patient-focused. This helps sponsors refine eligibility criteria, diagnostic pathways, and recruitment strategies for any planned trial. This is especially required in Hormone Receptor-Positive (HR⁺)/Human Epidermal Growth Factor Receptor 2-Negative (HER2⁻) metastatic breast cancer, where ever-evolving standards of treatment and therapies based on biomarkers make it essential that trials should closely reflect real-world practice.

HR⁺/HER2⁻ breast cancer: disease burden and distribution
Hormone Receptor-Positive (HR⁺)/Human Epidermal Growth Factor Receptor 2-Negative (HER2⁻) metastatic breast cancer refers to tumor cells that carry receptors for estrogen (ER) and/or progesterone (PR), which stimulate their growth, but do not overexpress the HER2 protein. The majority are of luminal subtypes: slow-growing Luminal A tumors have a good prognosis, whereas aggressive Luminal B tumors have a higher risk of recurrence. These are clinically relevant, as they determine treatment options.
Worldwide, HR⁺/HER2⁻ is the most frequent subtype of breast cancer, accounting for 65–75% of all cases. SEER reports indicate that more than 70% of U.S. cases are classified in this group, with comparable percentages in Canada and Europe. Incidence rates are increasing in Asia and Latin America as well because of lifestyle modifications and increased access to screening. While survival is usually improved in comparison to HER2-positive or triple-negative disease, the subtype’s biologic heterogeneity and threat of late recurrence remain a significant clinical challenge.
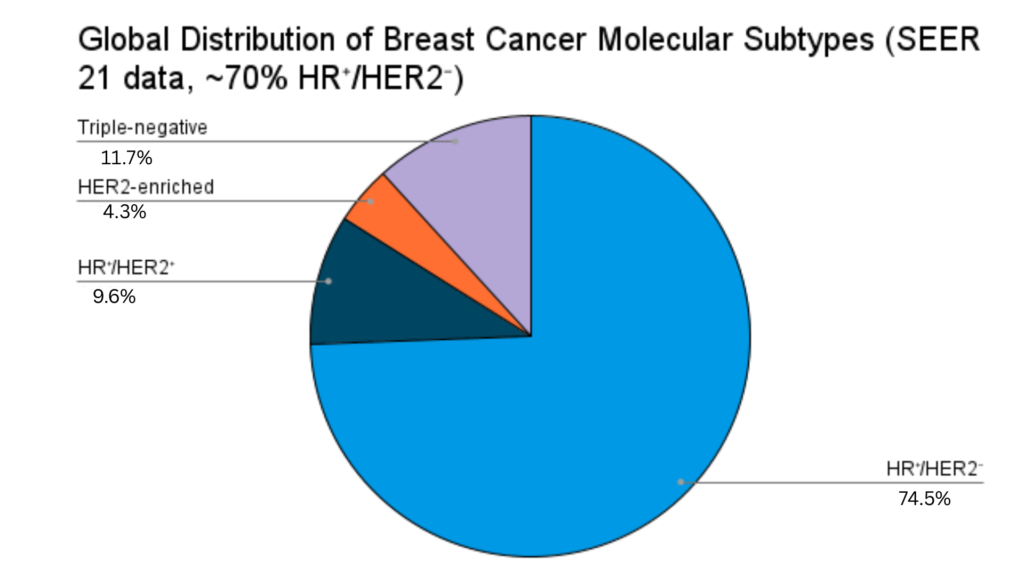
Figure: Pie chart showing the distribution of breast cancer subtypes within the SEER 21 registry. HR⁺/HER2⁻ accounts for approximately 70% of cases, while HR⁺/HER2⁺, HER2-enriched (HR⁻/HER2⁺), and triple-negative (HR⁻/HER2⁻) account for 9%, 4%, and 11%, respectively.
This article discusses the importance of HR⁺/HER2⁻ breast cancer trials for improved patient outcomes. It also outlines how early investigator engagement and KOL interaction can enhance trial design, facilitate recruitment, and improve long-term outcomes.
Why is conducting HR⁺/HER2⁻ breast cancer trials critical?
Despite available endocrine therapy, CDK4/6 inhibitors, and other targeted therapies, factors such as treatment resistance, shifting biomarker knowledge, and geographic differences in treatment of choice complicate trial design for HR⁺/HER2⁻ breast cancer. Here are the key challenges in an HR⁺/HER2⁻ breast cancer clinical trial:
- HR⁺/HER2⁻ breast cancer has a high prevalence rate globally.
- The treatment of HR⁺/HER2⁻ cancerous cells frequently involves long-term endocrine therapy with added chemotherapy, targeted therapy, and radiation, which is complicated and necessitates many studies.
- Another challenge is to identify distinct characteristics of the cancer, including precise hormone receptor status, tumor grade, and genetic makeup, facilitating an individualized treatment.
- HR⁺/HER2⁻ cancer cells may develop endocrine therapy resistance over time, particularly in metastatic or advanced cancer. This necessitates clinical trials to evaluate new approaches with combined therapies or new agents.
- High-risk subgroups in HR⁺/HER2⁻ breast cancer require targeted clinical investigation, highlighting the need for dedicated clinical trials.
These factors substantially underpin the critical importance of conducting clinical trials in HR⁺/HER2⁻ breast cancer to advance patient-centered treatment strategies. Currently, clinicaltrials.gov lists 489 trials for HR⁺/HER2⁻ breast cancer. Of these, 203 remain active, with 150 recruiting and 53 awaiting recruitment.
Current recommendations and standards of care for HR⁺/HER2⁻ breast cancer
With developments in endocrine therapy, targeted medicines, biomarker testing, and SERDs, the treatment of HR⁺/HER2⁻ metastatic breast cancer is changing quickly. Global practice is shaped by the American Society of Clinical Oncology (ASCO) and NCCN guidelines, which offer suggestions on treatment sequencing, comparator selection, and biomarker integration to guarantee that clinical trial designs are still applicable.
Key recommendations include:
- Comparators: Experts suggest using modern endocrine-based therapies with targeted agents as the new standard of care, replacing outdated comparators.
- For example, as a first-line treatment for HR⁺/HER2⁻ metastatic breast cancer, ASCO guidelines strongly advise combining an aromatase inhibitor (AI) with a CDK4/6 inhibitor rather than using traditional endocrine therapy alone.
- Biomarker-based interventions: There is a need to test ESR1 and PIK3CA key mutations that can inform treatment decisions and sequencing.
- Practice alignment: Protocols that include the usage of therapies in various patient groups and practice settings are recommended.
- Standardized testing and reporting: Suggests uniform biomarker tests and reporting to enhance the quality of trials and clinical judgment.
Challenges in designing global HR⁺/HER2⁻ breast cancer trials
Global trials in HR⁺/HER2⁻ breast cancer face specific challenges that may affect design, accrual, and generalizability:
- Regional variability in standards of care (SOC): CDK4/6 inhibitors, PI3K inhibitors, and genomic testing are widely available in high-income countries but limited in low- and middle-income regions. A single international protocol enables flexibility or regional stratification to remain practical.
- Assay harmonization and diagnostic integration: ctDNA-based ESR1 mutation testing can enrich and stratify patients, but adoption, access, and standardization remain obstacles. Well-defined laboratory plans, rigid quality-control procedures, and prearranged reporting schemes are critical.
- Enrollment dynamics and population heterogeneity: Post-CDK4/6 treatment settings are saturated with other competing phase III trials. Similar eligibility criteria, heterogeneous prior therapy, and patient age or tumor burden further restrict accrual and make interpretation more difficult.
- Comparator selection and acceptance: Investigator participation is affected by regional drug availability and practice behavior. Variability in comparators with local SOC can impede recruitment and protocol adoption.
- Equity and representativeness: Seniors, rural dwellers, and minorities are still underrepresented, and therefore, intentional inclusion strategies are intended.
How early site & investigator engagement enables effective trial design
Early interaction with investigators helps overcome key operational and scientific hurdles in HR⁺/HER2⁻ breast cancer trials
- Align comparators and eligibility with local SOC: Discussions with investigators and key opinion leaders (KOLs) help sponsors avoid inappropriate control arms. They also align eligibility criteria with regional treatment differences..
- Active feedback on protocol design: Involving investigators early provides valuable protocol feedback, ensuring study design aligns with real-world clinical practice.
- Plan biomarker testing capacity: Facilitates obtaining information early from local laboratories to carry out assays such as ESR1 or PIK3CA.
- Assess enrollment strains: Researchers conduct site-level feasibility surveys to evaluate competition and realistically recruit potential participants.
- Design patient-friendly operations: Investigator feedback plays a key role in shaping pragmatic trial activities. These include visit frequency, imaging schedules, and the use of remote or decentralized alternatives.
- Reduce inequities: Start early dialogue with investigators in underserved communities. Involve patient advocates to develop outreach strategies and supportive infrastructure.
- Design achievable inclusion and exclusion criteria: Early investigator input helps avoid overly strict criteria that slow participant recruitment.
Strategic approaches to HR⁺/HER2⁻ trial design through early site and investigator engagement
Balanced execution of real-world feasibility and scientific precision is essential to conduct successful HR⁺/HER2⁻ breast cancer clinical trials. Currently, clinicaltrials.gov lists 489 trials for HR⁺/HER2⁻ breast cancer. Of these, 203 remain active, with 150 recruiting and 53 awaiting recruitment. The following strategic approaches outline how sponsors can design and implement effective clinical trials:
- Comprehensive landscape assessment: Follow the current guidelines from ASCO, NCCN, and ESMO; prepare competing protocols; and conduct adequacy studies with KOL and investigators.
- Engage with sites and investigators from the start: Use real-world advisory boards to streamline high-volume site engagement. Adjust inclusion and exclusion criteria early. Align protocols with site needs. Integrate clinical practice into trial design for smoother execution.
- Protocol refinement: Facilitate patient participation with streamlined criteria, minimize exclusion filters, and, when suitable, include biomarker-driven arms to increase participation.
- Patient-centered recruitment and retention: Education initiatives via community oncologist partnerships and digital engagement, alongside enhanced remote monitoring and streamlined scheduling to increase trial participation.
- Compliance ethics and regulatory requirements: Streamline multicenter sub-regional applications, integrate patient-reported outcomes, and resolve ethics early to preempt regulatory roadblocks.
Conclusion
Effective early investigator communication is key to successful clinical trials, especially in difficult-to-enroll groups like HR⁺/HER2⁻ breast cancer. Engaging investigators early allows sponsors to align trial design with local clinical practice. This improves recruitment and compliance with local standards. Early collaboration also supports enrollment of more representative patients. As a result, trial data quality and outcomes improve across diverse regions, treatment patterns, and patient responses.
Are you planning a clinical trial in HR⁺/HER2 and aiming to avoid unexpected challenges by early site & investigator engagement?
Are you planning a clinical trial in HR⁺/HER2⁻ breast cancer and want to avoid unexpected challenges? Early site and investigator engagement can help you streamline site selection, improve recruitment, and align with global standards. Fill out the form below to discuss your trial plans with our experts.