Conducting Colorectal Cancer Clinical Trial? Here Is Why You Should Engage Investigators In Canada

Colorectal cancer (CRC), also known as bowel cancer and colon cancer, is the development of cancer from the colon or rectum.
Globally, colorectal cancer is the third most common type of cancer in both men and women making up about 10% of all cases. Currently, there is a significant focus on the development of drugs for the treatment of CRC.

Signs and symptoms of colorectal cancer include blood in the stool, a change in bowel movements, weight loss, and feeling tired all the time. It typically starts as a benign tumor, often in the form of a polyp, which over time becomes cancerous.
Colorectal clinical trials around the world
A total of 5,412 studies on Colorectal Cancer have been recorded around the world, with most trials being in Europe(1,823), North America (2,284), East Asia (893), and Pacific regions (135). (Clinicaltrials.gov)
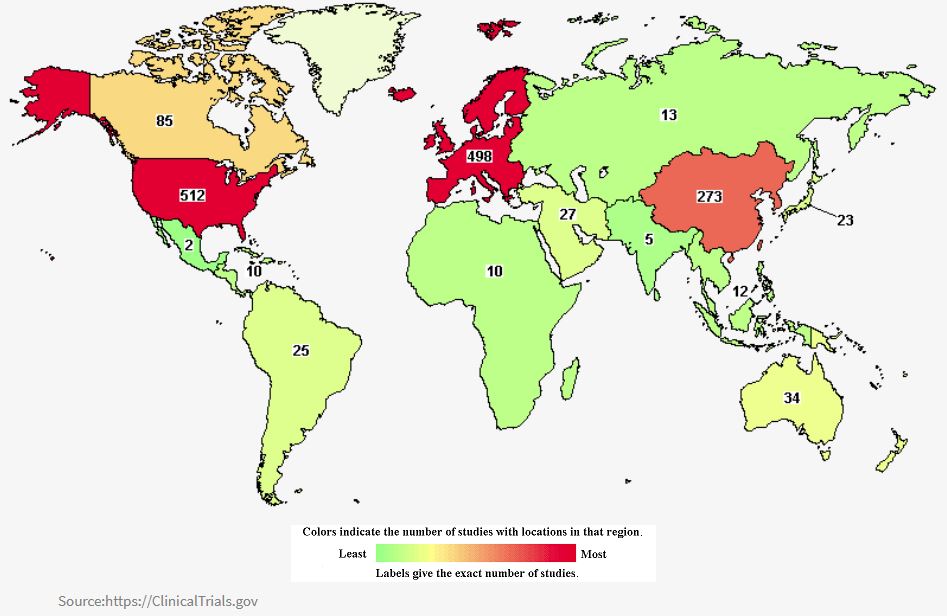
Currently, ongoing studies of colorectal cancer around the world are 1,610, particularly in North America (671), Europe (593), East Asia (364), and the Pacific region (43).
OGD (USFDA) requires clinical trials on colorectal cancer drugs
Even for generic drug products, like
- Capecitabine
- Tipiracil Hcl, Trifluridine
- Regorafenib
those used in the CRC treatment, OGD requires a clinical trial to be conducted.
Thus, applicants intending to submit for marketing approval of these drug products are required to conduct a clinical trial in CRC patients.
With generic drugs also requiring clinical trials in CRC patients (along with so many companies going for such clinical trials), there is a significant need to identify great sites and investigators, who can provide good quality data with faster recruitment.
Colorectal cancer phase 2-3 Clinical trial feasibility
In a recently concluded clinical trial feasibility in Colorectal cancer patients on Credevo, the preliminary recruitment rate was assessed in Canada and Lithuania.
The study required
– Patient with non-resectable metastatic colorectal cancer with histological or cytological documentation of adenocarcinoma of the colon or rectum
– Patient in third-line or fourth-line treatment for metastatic colorectal cancer
Several investigators participated in this feasibility. Here are the salient results from the assessment.
1. Canadian investigators were more responsive than those in Lithuania.
2.Average recruitment rate, reported by Canadian investigators, was 3.5 patients per site per month.
3. Highest recruitment rate reported was 5 patients per site per month.
Reasons to engage investigators/sites for CRC clinical trials in Canada
There are several.
- Canada has sites with both private as well as public ownership, allowing you to take benefits of both sides.
- Canadian investigators are definitely very qualified, experienced, and, most importantly, quite motivated to engage in clinical research.
- The feasibility of any good clinical trial can be conducted quickly with active participation from site staff and investigators.
- A recruitment rate of 3.5 – 5 patients per month is possible for CRC trials, making it one of the most favorable prospective rates.
- Canadian regulatory process is quite smooth, well defined, and quite predictable. This makes it a lot easier to define projections for approvals and related timelines. Click here for details on the Canadian regulatory process
- The regulatory approvals process is parallel for regulatory authority and ethics committee, saving some significant time.
- A quite convenient regulatory approval time of 30 days.
- Easy regulations for the import of study medications.
- Easy study start-up and management.
Contact investigators from this feasibility assessment or Get any other support for conducting such clinical trials
Would you like to contact these investigators and assess feasibility with them for your clinical trial? or Do you need any other support for conducting such clinical trials?
Now, it’s very easy to do so.
Just provide your requirement details & email below. Don’t worry, your email will not be published or shared.
For other details, contact us at helpdesk@credevo.com.
References
- https://www.cancer.org/cancer/colon-rectal-cancer/treating/chemotherapy.html
- https://www.mayoclinic.org/diseases-conditions/colon-cancer/symptoms-causes/syc-20353669
- https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2017-2019.pdf
- https://clinicaltrials.gov/ct2/results/map?recrs=adf&cond=Colorectal+Cancer&map=
One thought on “Conducting Colorectal Cancer Clinical Trial? Here Is Why You Should Engage Investigators In Canada”
Comments are closed.