Clinical Trial Regulatory – Ukraine

Ukraine, the eighth largest European country by population, has often been overlooked as a clinical trial site, yet remains an attractive location for multinational companies for its clinical studies potential.

Ukraine is one of the most attractive regions of the post-Soviet area in terms of clinical research because of its
- Vast population which is concentrated in several large cities,
- Well equipped investigative sites,
- Qualified and GCP-trained medical personnel, as well as a
- favorable legal landscape with Regulations compliant with the European standards. [1]
Clinical Trials in UKRAINE
Ukraine clinical trials legislation is completely harmonized with respective directives, guidelines and bylaws of the European Union and recognized as the most advanced of all Commonwealth of Independent States (CIS) countries.
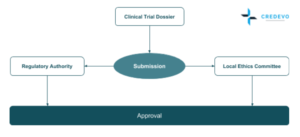
Regulatory Authority (RA) & Local Ethics Committee (LECs) submissions are done in Parallel and all approvals are obtained within 57 calendar days where the study start-up can be achieved in 12-16 weeks total, comparable to most EU countries and all these can be achieved at cost effective range. [2]
Advantages for conducting Clinical Trials in UKRAINE?
- High quality of data confirmed by numerous FDA/EMA audits.
- A large pool of highly motivated, experienced, and GCP-trained clinicians.
- A large pool of potential study subjects, with recruitment rates higher than those of the U.S. or EU countries.
- Public healthcare system comprising over 2,500 public healthcare facilities with International standards for medical care and a centralized system of healthcare that aids recruitment.
- Major improvements in legislative and regulatory environment over the past years and efficient approval process that compares favorably to other countries. [2][3]
Leading therapeutic areas
- Heart disease
- Stroke
- HIV/AIDS
- Liver disease
- Lung Disease
Language
The application should be completed in English, Russian, and Ukrainian. The official guidelines state the labels on IMPs and draft informed consent should be in Ukrainian. The protocol summary should be in Ukrainian.
Regulatory Authority
Clinical trials in Ukraine are regulated and approved by the Ministry of Health (MoH). The department under MoH which reviews CTAs is the “State Pharmacological Center”, also known as the “State Expert Center” or simply, the “center”.
Clinical trials may only be conducted at approved Health Care Settings (HCS). In order to be approved, HCS must have a license for medical practice and a MoH issued accreditation certificate.
Ethics Committee
Ukraine only has Local ethics committee (LEC) known as Health Care Settings (HCS). Approval is required from each HCS where the trial will be conducted. The LEC of the HCS will be an overseeing body throughout the trial.
Officially the LEC has 30 days to review, with one clock-stop to request additional materials, the applicant will have 30 days to provide the necessary materials.
The LEC will inform the applicant of their decision in writing. For a negative result the sponsor may submit again within 30 days, with the LEC completing the review within 30 days.
Investigators & Sites
Investigators must work in the HCS where the trial is to be conducted or if they work for an institute of higher education, a contract between the institute and HCS must be completed. Investigators in Phase 1 must have special knowledge and expertise relevant to the trial.
Requirements for CTA submission
- Cover letter
- Application for getting conclusion of the State Expert Center MoH Ukraine/endorsement of the ethics
- Committee at HCS pertinent to conducting clinical trial of medicinal product
- Protocol of the clinical trial of the medicinal product with all amendments to it that should include the
- Information stated in Section 6 of the Good Clinical Practice.
- A brief summary (synopsis) of the protocol in Ukrainian.
- Case report form (except for international clinical trials).
- Investigator’s Brochure that should include an information stated in section 7 of Good Clinical Practice (GCP).
- An investigational medicinal product dossier.
Approval Process
- Three copies of the CTA should be submitted to the center along with the fee and One copy of the CTA should be submitted in e-format to the LEC. Click here for application format
- A contract must be signed between the sponsor and the center for conducting the expert evaluation, click here for contract form and all the formats for various formats can be found on the Department for Expert Evaluation website
- The English version of centre website is available but, however most of the information is literally lost in translation, when using the English site. The translate feature of browsers (e.g. Google Chrome) is the most efficient way to access the information.
- All the pertinent guidelines and regulations can be found in the document library or on the Department for Expert Evaluation of Materials of Preclinical and Clinical trials (English Version) and Department for Expert Evaluation of Materials of Preclinical and Clinical trials (Ukranian Version)
- The full dossier of investigational medicinal product shall be submitted in a given format if the investigational medicinal product hasn’t been registered in Ukraine. Please click here for the the format.
- If a medicinal product has already been registered in Ukraine the sponsor may submit a summary product characteristics as an investigational medicinal product dossier.
- For comparators and placebo the simplified dossier or summary product characteristics shall be submitted.
- As in most countries, as soon as one of the reviewing bodies asks the sponsor for more information or clarification, the clock stops. Once the sponsor’s response is received, the deadline countdown resumes.
- Also, the authorities can issue comments only once. Response may be provided informally, or in a formal letter.
- If there are no inquiries, it is possible to achieve full approval in 50 days, on average. However, when queries are involved, approvals take much longer.
- After approval and trial initiation, the MoH must be notified within 8 days after the start of a trial, when the first patient signs informed consent. [2][3][4]
Type of Approval
The Local Ethics Committee (LEC) review can be before, in parallel, or after the MoH review.
Approval Timeline
Official estimates state a 50 day for review, with a one-time clock stop for the center to request more information. If more information is requested, the sponsor has 60 days to respond, if not the CTA will be withdrawn. If everything looks fine must then be authorized by the Ministry of Health within 10 days.
The sponsor has 30 days to appeal a negative decision to the MoH, the appeal will be considered within 60 days and the entire process from approval to site start-up is around 4-5 months. [4]
Fee
The fee for application review is 3300 Euros when the investigational drug is not registered in the country of origin and 2000 Euros if it has been and no fees for LEC review. [4]
Import/Export Licence
Approval of a trial by the MoH grants automatic permit for importation of clinical trial drugs. Importing IMPs will still require the go ahead from the importer of record.
However, the import/export of narcotic products, psychotropic substance, or precursor, in Ukraine still require the appropriate permit from the MoH – State Service of Ukraine on Drug Control.
Clinical Trial Statistics
A total of 1,804 clinical Trial studies were registered in Ukraine with 472 Ongoing studies with major studies in therapeutic areas like Immune disease followed by Respiratory, Liver, and Heart disorders. [5]
Need more info or Support?
Feel free to contact us with your questions or if you need any support in Ukraine.
Reference
- http://www.jforcs.com/wp-content/uploads/2015/05/10.-Drugs-Marketing-Authorisation….pdf
- https://www.pharm-olam.com/region/conducting-clinical-trials-ukraine
- https://cdn2.hubspot.net/hubfs/4238150/PharmOlam_March2018/PDF/clinical-trials-in-ukraine-whitepaper.pdf?t=1526034567095
- https://www.nihcollaboratory.org/sites/CbyC/Pages/CSIGovAg.aspx?Country=Ukraine
- https://clinicaltrials.gov/ct2/results?cond=&term=&cntry=UA&state=&city=&dist=