Hong Kong – How To Utilize Clinical Trial Regulatory Process Effectively?

Hong Kong has a impeccable history of international research with impressive track record of conducting global clinical trials. It has an outstanding healthcare system and indicated as one of the lowest infant mortality rates and the longest life expectancy in the world.

Hong Kong is an emerging market for clinical trials. It is the world’s eighth largest freest economy country. Hong Kong provides excellent infrastructure and well-established research institutes, strong presence of academic institutions, and high-quality investigators. The majority of clinical trials conducted in Hong Kong are in Phases II to IV.
Although Hong Kong is a Special Administrative Region of China, Clinical trial regulations differ from China. It does not follow the same medical device or pharmaceutical regulations as the mainland.
Advantages
- Hong Kong data can be used to file New Drug Application in CFDA
- Fast approval timeline approx 3-6 months
- Presence of good academic institutions
- Quality investigators
- Strong government support for clinical research
Hong Kong in Statistics
A total of 1,801 Clinical Trials were registered with 623 On-Going Trials. Major therapeutic areas most of the trials registered are
- Gastrointestinal and digestive disorders
- Oncology
- Respiratory diseases
- Hepatic diseases &
- Communicable diseases.
Regulatory body
Clinical trials in Hong Kong are regulated by the Department of Health (DH). Certificate for Clinical Trial/Medicinal Test (certificate) is required for the purpose of conducting a clinical trial on human beings. Pharmacy and Poisons Board of Hong Kong is the statutory body to issue the certificate.
An applicant should first receive approval from the hospital’s Ethics Committee (EC) before applying for. Obtaining EC approval generally takes 4 to 6 weeks.
English and Chinese are both official languages of Hong Kong. All electronic medical data is in English. The patient consent forms can be in both English and Chinese but Chinese is required.
Ethics approval
- Ethics Committees in Hong Kong follow International Council of Harmonization(ICH) principles. Hong Kong ethics committees are established in clusters. There are six ethics committees covering the 41 hospitals in Hong Kong. The ethics committees meet every 2 weeks. Each cluster regional ethics committee (“REC”) will have its own requirements and application processes.
- The establishment of the Clinical Research Ethics Committee of the Hong Kong Doctors Union is a major milestone. The Committee is empowered to issue IRB approval for research conducted in private practice. Once IRB approval is obtained, principal investigators can apply for a clinical trials certificate from the Department of Health.
- Applicant has to create an account in HA CRER portal before submitting documents for clinical research ethics review.
- When a new application is made, secretary will arrange a panel meeting, reviewers will review the application and documents. All the comments made by the reviewers are gathered and final decision is made by the secretary.
- The whole process usually takes around 12 weeks.
Regulatory approval requirement & process
Application Requirements
- Protocol
- Investigator’s brochure
- Pre-clinical study results
- Drug sample
- EC approval letter
- CV of the PI
- Documentation of Ethics Committee approval
- The proposed patient information and consent, in both English and Chinese, or in Chinese only
- Evidence that the trial medication is manufactured in accordance with good manufacturing practices (GMP) (e.g., a copy of the manufacturers GMP certificate)
- A sample certificate of analysis of the drug
- If the trial involves Chinese medicines, a letter from a Chinese medicine practitioner participating in the clinical trial is also required
Process
- To apply for clinical trial certificate, applicant can submit the duly completed application form and required documents by post or by hand at Drug Registration and Import/Export Control Division office.
- Applications are categorized into two schemes
- Standard Scheme (When the sponsor of a clinical trial is a pharmaceutical company or research organisation/institution) – Trials involving drug registered in Hong Kong
- Listed Scheme (Clinical trials initiated and conducted by a sponsor-investigator) – Trials involving drug not registered in Hong Kong
- Who can be the applicant?
- For Standard Scheme – A local company holding relevant licence(s) or the principal investigator who conducts the trial should be the applicant.
- For Listed Scheme (Trials involving drug not registered in Hong Kong) – Only the sponsor-investigator who initiates and conducts the trial should be the applicant
- For Listed scheme the sponsor-investigator should conduct a risk assessment to grade the clinical trial into one of the three categories (i.e. Type A, B or C) by taking into consideration the registration status of the drug.
- Type A: No higher than the risk of standard medical care
- Type B: Somewhat higher than the risk of standard medical care
- Type C: Markedly higher than the risk of standard medical care.
- Application form can be downloaded from http://www.drugoffice.gov.hk/ website or
- Standard Scheme Application form
- Listed Scheme Application form
- Application should be submitted in person or by mail to the Drug Registration and Import/Export Control Division of Drug Office. Applicant will be acknowledged by receipt upon payment of the application fee (currently HK$1,420). The acknowledgement receipt contains an application number.
- IRB approval document can be submitted later, but the applicant can get the final clinical trial approval only after submitting the IRB approval document
- If the application is approved, the applicant will be notified in writing. Payment of
the certificate fee (currently HK$1,420) and collection of certificate should be made in person. - Enquires about the application process or Progress of the application can be made at Drug Registration and Import/Export Control Division of the Drug Office or Phone (852) 2319 8458.
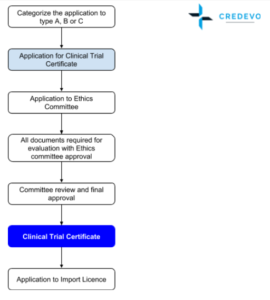
Approval Timelines
Government agency approval takes 4 – 6 weeks with additional one week along with Import licence. Overall previous approval timelines suggests 9 – 16 weeks
Ethics committee approval is required before getting approval from government.
Application Fee
If application is approved, the applicant is required to pay certificate fee of HK$1,420 to obtain the certificate.
Type of approval
Application to the IRB/IEC is Parallel with the regulatory submission, as the final regulatory approval will not be granted until IEC/IRB approval has been received.
Import & Export Licence
- If the investigational product is being imported into Hong Kong an import license from the Trade and Industry Development Office will be required.
- The import licence application for pharmaceutical products & medicines should be completed in quadruplicate or the export licence application completed in triplicate, should be lodged in Drug Registration and Import/Export Control Division office.
- A numbered receipt will be issued to the applicant and applicants shall collect the processed applications after one working day at the Drug Import/Export Control Unit with the receipt.
- The applicant will be given the original and duplicate of licence
- In the case of an Import Licence application, the applicant will be given the original and duplicate of licence.
- In the case of an Export Licence application, the licensee will be given only the original, which should be surrendered to the carrier.
- Application for import and export licences for unregistered pharmaceutical products for re-export purpose can only be made through the electronic system, Pharmaceuticals Licence Application and Movement Monitoring System (PLAMMS).
- If a sponsor plans to conduct the clinical trial in both Hong Kong and mainland China, the application in Hong Kong must include either the approval from the China Food and Drug Administration (“CFDA”) or the study protocol which was submitted to the CFDA.
- Applications for import licence Form 3, export licence Form 6 covering pharmaceutical products and medicines, import and export authorizations covering controlled chemicals are free of charge.
Medical Device Regulatory
Hong Kong’s Department of Health (DH) currently has a voluntary registration system under the Medical Device Administration Control (MDACS). All medical devices used in clinical trials are to be registered with the DH except for certain medical device and the Director of Health will issue a Certificate for Clinical Trial to legally allow the medical device to be used in a clinical trial.
Medical devices in Hong Kong are categorized into four classes by risk level, depending upon on the design and intended use of the device.
- Class I medical devices are considered low risk,
- Class II and III medical devices are considered medium risk, and
- Class IV medical devices are considered high risk.
Need Support in Hong Kong or Have questions?
We’d love to help you conduct clinical trials in Hong Kong.
Connect with experienced and resourceful sites, services providers and experts in Hong Kong.
Provide your details below.