Singapore Clinical Trial Regulatory Process

Singapore has a well-established regulatory framework for clinical trials, overseen by the Health Sciences Authority (HSA). The HSA ensures that clinical trials conducted in Singapore adhere to ethical principles and Good Clinical Practice (GCP) guidelines.
This article was last updated on 14/03/23.

Singapore is considered as the R&D pioneer in Asia with highly qualified clinicians, a solid and advanced infrastructure, cutting-edge technology, and commitment by the government to become one of the preferred clinical research destinations worldwide.
Singapore is central to the region (South East Asia) and hub of the life sciences industry, acting as a pacesetter to carry out further development across the rest of Asia as an investigation candidate moves through the later phases. Conducting a Phase 1 study in Singapore can help an R&D company learn about the intricacies of performing development activities in Asia.
Why choose Singapore for your clinical trials?
- Strategic Location: Singapore is situated in the heart of Southeast Asia, making it easily accessible from major Asian cities. Its central location allows for efficient coordination of multinational clinical trials across the region.
- Robust Healthcare Infrastructure: Singapore boasts world-class healthcare facilities and research institutions, including hospitals, research centers, and universities. These institutions provide state-of-the-art equipment, expertise, and support services necessary for conducting clinical trials.
- Regulatory Environment: Singapore has a well-established regulatory framework for clinical trials overseen by the Health Sciences Authority (HSA). The regulatory process is known for its efficiency and transparency, with clear guidelines and timelines for approval.
- Ethical Standards: Singapore upholds high ethical standards in clinical research, ensuring the safety and welfare of trial participants. Institutional Review Boards (IRBs) or Ethics Committees review and approve clinical trial protocols to ensure they meet ethical guidelines.
- Multicultural Population: Singapore’s diverse population, comprising various ethnicities and backgrounds, makes it an ideal location for conducting clinical trials with broader demographic representation. This diversity enhances the generalizability of trial results.
- Skilled Workforce: Singapore has a highly educated and skilled workforce, including researchers, clinicians, and clinical trial professionals. Access to this talent pool facilitates the smooth conduct of clinical trials and ensures high-quality data collection and analysis.
- Stable Political and Economic Environment: Singapore offers a stable political and economic environment conducive to conducting long-term clinical trials. Its strong governance, rule of law, and intellectual property protection provide confidence to sponsors and investors.
- Technological Advancements: Singapore is at the forefront of technological innovation, with initiatives to incorporate digital health solutions and advanced technologies into clinical trials. This includes telemedicine, wearables, and data analytics, which can streamline trial processes and enhance data accuracy.
- Government Support: The Singaporean government actively promotes biomedical research and development through various initiatives, grants, and incentives. This support encourages collaboration between industry, academia, and government agencies, fostering a conducive environment for clinical trials.
- Quality of Life: Singapore offers a high quality of life for researchers, sponsors, and trial participants. Its modern amenities, efficient public transportation, safety, and healthcare services contribute to a positive experience for those involved in clinical trials.
Phase I studies
- The country plays an impeccable role in conducting Phase 1 studies in the Asia-Pacific region.
- Many pharmaceutical and biotech companies are now choosing to bring their innovative early-phase trials to Singapore over other conventional options in the United States or Europe. This is largely due to the track record of success stories during the last five years.
Clinical trial regulatory authority in Singapore
The Health Sciences Authority (HSA) regulates the conduct of clinical trials of therapeutic products and medicinal products under the Health Products (Clinical Trials) Regulations and the Medicines (Clinical Trials) Regulations respectively.
The regulations require sponsors of clinical trials to submit details of the clinical trials to HSA under one of 3 submission routes.
| Category | Application Type |
|---|---|
| Clinical trials of therapeutic products | Clinical Trial Authorisation (CTA) |
| Clinical trials of therapeutic products used by approved label | Clinical Trial Notification (CTN) |
| Clinical trials of medicinal products | Clinical Trial Certificate (CTC) |
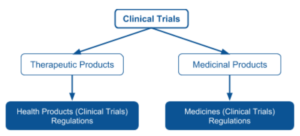
However, Medical device clinical trials are not regulated by the HSA.
Observational clinical trials, Class 1 CTGTP trials, and medical device trials are nevertheless required to comply with the requirements of the Human Biomedical Research Act.
What are the regulatory requirements for clinical trials?
For clinical trials of therapeutic products (e.g. pharmaceutical drugs and biologics), clinical trial authorization (CTA) or acceptance of clinical trial notification (CTN) is required before the trial is initiated or conducted.
For clinical trials of biological products like cell, tissue, and gene therapy products or complementary health products a clinical trial certificate (CTC) is required before the trial can be initiated or conducted.
Clinical trials must be conducted in compliance with the ICH E6 Good Clinical Practice guidelines along with the Medicines (Clinical Trials) Regulations for therapeutic products & Health Products (Clinical Trials) Regulations for Biological products.
Documents required for various categories
| No. | Documents | Clinical Trial Authorization (CTA) | Clinical Trial Notification (CTN) | Clinical Trial Certificate (CTC) |
| 1 | Clinical Trial Protocol | ✔️ | ✔️ | ✔️ |
| 2 | Informed Consent Form (English) | ✔️ | ✔️ | ✔️ |
| 3 | Investigator’s Brochure | ✔️ | – | ✔️ |
| 4 | List of Overseas Trial Site, where applicable | ✔️ | ✔️ | ✔️ |
| 5 | Principal Investigator’s CV | ✔️ | ✔️ | ✔️ |
| 6 | Good Manufacturing Practice (GMP) Certificate | ✔️ | ❌ | ✔️ |
| 7 | Certificate of Analysis (COA) for study batches of Investigational Products | ✔️ | ❌ | ✔️ |
| 8 | Chemistry. Manufacturing and Control (CMC) information, if requested by HSA | ✔️ | ❌ | ✔️ |
| 9 | Approved Product Label | ❌ | ✔️ | ❌ |
| 10 | IRB Approval Letter | ❌ | ✔️ | ❌ |
What are the regulatory requirements for First-in-Human (FIH) trials of therapeutic products?
The therapeutic product need not be approved in other countries before the FIH trial can be conducted in Singapore. As with all clinical trials, the sponsored applicant must be a locally registered company.
For guidance on non-clinical and clinical requirements, companies may refer to applicable ICH guidelines and relevant guidelines issued by the major agencies (FDA, EMA) [4]
What is the process of approval?
- A guidance document gives information about whether a drug belongs to the CTA or CTN category
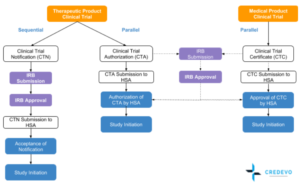
- Once it has been determined that a clinical trial on a therapeutic product/s is subject to the requirements of a Clinical Trial Authorisation (CTA) or Clinical Trial Notification (CTN), the sponsor should submit the clinical trial application to HSA.
- Clinical trials of medicinal products like cell, tissue, and gene therapy products or complementary health products are regulated under the Medicines Act and Medicines (Clinical Trials) Regulations. Such clinical trials require a Clinical Trial Certificate (CTC) issued by HSA before the trial can be conducted
- The sponsor should be a locally registered business entity registered with the Accounting and Corporate Regulatory Authority (ACRA) in Singapore. The sponsor should submit the clinical trial application to HSA online via the Pharmaceutical Regulatory Information System (PRISM).
- For clinical trials that require clinical trial authorization (CTA) or a clinical trial certificate (CTC), the clinical trial application can be submitted concurrently to HSA and the relevant IRB. For clinical trials that require clinical trial notification (CTN) to HSA, the submission should be made only after having received IRB approval for the clinical trial.
- Subsequent submissions like Substantial amendments, Serious Breaches, Trial Status Reports, etc to HSA may be required during a clinical trial. All subsequent submissions to HSA should be submitted via PRISM unless otherwise specified.
- When the sponsor gets approval from HSA, can initiate the trial.
- The authorization (for CTA), or acceptance of notification (for CTN), of the clinical trial by HSA, is valid for the duration of the clinical trial. The duration of the clinical trial refers to study initiation to study completion. [3]
Process Flow
What is the timeline for approval?
The timeline varies with the category to which the trial belongs timelines for various categories are
- Clinical Trial Authorisation (CTA) – 30 Working Days
- Clinical Trial Notification (CTN) – 5 Working Days
- Clinical Trial Certificate (CTC) – 30 Working Days
For CTA Phase 1 clinical trials solely to evaluate bioequivalence, bioavailability, food effect or drug-drug interaction take around 15 days.
Fee for regulatory
There are currently no fees imposed for the submission of a clinical trial application to HSA.
Import & Export license
- The import and supply of therapeutic products, medicinal products, and medical devices for clinical research in Singapore are regulated under the Health Products (Therapeutic Products as Clinical Research Materials) Regulations, the Medicines (Medicinal Products as Clinical Research Materials) Regulations, and the Health Products (Medical Devices) Regulations respectively.
- Under these regulations, notification to HSA (CRM – Clinical Research Material Notification) is required before the import of overseas-manufactured CRM, or before the supply of locally-manufactured CRM by the local manufacturer. Besides, local manufacturers, importers, suppliers of CRM, and clinical trial sponsors are required to comply with duties and obligations under the regulations.
- Sponsors of regulated clinical trials submit the CTA/CTN/CTC application form including the CRM notification.
- Sponsors of non-HSA-regulated clinical research may be required to endorse the CRM notification that is to be submitted by the importer or local manufacturer.
- An export license is not required from HSA for the shipping of biological samples overseas for testing. [6]
Do you need regulatory support in Singapore?
Credevo provides complete regulatory support for your drug development. Provide your requirement details below to talk with us
One thought on “Singapore Clinical Trial Regulatory Process”
Comments are closed.