Malaysia – Why and How to Start Your Clinical Trials?
Malaysia is one of the world’s most popular countries for coastal tourism, known for its culinary sensations, beautiful beaches, idyllic islands, and national parks. Conducting clinical trials in Malaysia offers a diverse patient population, well-established healthcare infrastructure, favorable regulatory environment, and cost-effective research opportunities.
Note: This article was updated in May, 2023

Malaysia owns a large and diverse multi-ethnic population along with a strong two-tier health care system. Interestingly, life expectancy/infant mortality rates are comparable in the United States and European countries.
Top reasons to choose Malaysia for your clinical trials
There are many compelling reasons to consider Malaysia for your clinical trials, here are a few of them
- Competitive costs and an established healthcare system compared with other regions.
- Great infrastructure in specialties like
- Cardiology,
- Gastroenterology,
- Respiratory,
- Oncology, and
- Endocrinology.
- With improving standards of living, Malaysia is seeing a higher incidence of lifestyle diseases, along with communicable diseases in rural areas.
- Government-supportive regulations to make Malaysia a preferred clinical research destination
- A streamlined and agile regulatory system
- Regulatory and ethics submission language can be made in English
- Parallel and fast approval timelines
- Quick start-up timelines
- Lower per-patient costs and Institutional Review Board (IRB) fees compared to most countries
- Medical device and non-interventional studies require only Institutional Ethics Committee (IEC) approval
- Most investigators are trained in Good Clinical Practice (GCP) and have the experience to conduct clinical trials
- The launch of Phase 1 clinical trial guidelines, has enabled Malaysia to conduct early phase trials in drug discovery and development
The landscape of the Malaysian population
- 32 million people, of which Malays and Indigenous (60%), Chinese (28%), Indian (8%), and others
- 72.8% reside in urban centers
- 95.4% literacy rate
Malaysia has a wide ethnic population as above, which helps in testing the drug in multiple ethnic groups.
Malaysian government initiatives
The government of Malaysia, with an aim to promote and increase the number of clinical studies in the country, established Clinical Research Malaysia (CRM) in 2012.
The objectives were to effectively increase the speed, reliability, and delivery of outcomes.
Clinical trial status in Malaysia
A total of 1,881 clinical trials are conducted in Malaysia with 434 ongoing studies according to clinicatrials.gov. (Data as of Sep, 22), and this shows the interest of researchers to perform their clinical trials in Malaysia.
Therapeutic areas for clinical trials in Malaysia
Currently, the major therapeutic areas to perform clinical trials in Malaysia are
- Heart disease and Stroke
- Influenza and Pneumonia
- HIV/AIDS
- Tuberculosis
- Diabetes
Recent achievements of the clinical trials in Malaysia
- Clinical trials of new Hepatitis C combination treatment held in the country have shown not only a high 97% cure rate but also better efficacy in treating the most severe form of Hepatitis.
- Hospital Miri, Sarawak has been the 2nd top recruiter globally for a respiratory study recently.
- Hospital Kuala Lumpur was among the top 2 recruiters for a Cancer Registry study in Asia.
- The government signed MoUs with CROs to attract more global trials into the country.
Need support to conduct your clinical trial in Malaysia?
Credevo offers expertise in clinical trial regulations, drug products & nutraceutical/health foods registration, and many more services in Malaysia. Provide your details in the below form to connect with us and explore our services.
Regulatory for clinical trials in Malaysia
- There are mainly two agencies regulating clinical trials in Malaysia.
- Apart from this, agencies reviewing the clinical trials are
- Institutional Review Board (IRB), including the Medical Research & Ethics Committee (MREC) for trials using the Ministry of Health (MOH) Malaysia facilities or
- Local IRBs for non-MOH facilities.
The National Pharmaceutical Regulatory Agency (NPRA) ensures the quality, efficacy, and safety of pharmaceuticals. Additionally, it also acts as a secretariat to the Drug Control Authority (DCA), which
- reviews the matters related to product registration, and
- approves or rejects the application for a clinical trial import license (CTIL) or clinical trial exemption (CTX).
Clinical Trial Import Licence (CTIL)
A clinical trial import license is necessary to import any product for clinical trials.
Clinical Trial Exemption (CTX)
Clinical Trial Exemption (CTX) is the authorization to manufacture any product/s solely to produce samples for clinical trials.
Locations and clinical trial sites in Malaysia
- Sponsors and CROs can conduct clinical trials in Malaysia at ministry establishments, private hospitals, and medical teaching hospitals.
- There are well-established medical centers and sites in Malaysia, which are ranked top in conducting and recruiting clinical trials.
Ethics Committee
The Institutional Review Board (IRB) structure in Malaysia depends on the location or type of facility conducting the research.
- Most university hospitals have their own local IRB/IEC, while research conducted at the Ministry of Health Hospital falls under the purview of the central IRB.
- There are 13 NPCB-registered IRBs/IECs in Malaysia.
- These IRBs/IECs include the Ministry of Health Medical Research and Ethics Committee (MOH MREC), the Penang Ethics Committee, and ethics committees from universities and private hospitals.
- The IRB/IEC of the respective sites approves the conduct of clinical trials.
- The MOH hospital sites have only one central IRB which is the MOH-MREC.
- Applicants can submit the applications for IRB approval online via the National Medical Research Register.
Fee for the Ethics Committee (EC) review
- At present there is no fee required for Ethics Committee (EC) review.
- The processing fee per product is RM500.
Clinical trial registration
- All the clinical trials that require CTIL/CTX, need to register with the National Medical Research Register (NMRR).
- The applicant needs to obtain a unique full NMRR registration number from the NMRR website before submitting the CTIL/CTX application to NPCB,
- The applicant must use the NMRR registration number in all communications made with the National Pharmaceutical Control Bureau (NPCB).
Who can apply for CTIL/CTX?
The following people are eligible to apply for the CTIL/CTX
- An investigator,
- An authorized person from a locally registered pharmaceutical company/ sponsor/CRO with a permanent address in Malaysia, or
- A sponsor or CRO without an office in Malaysia can outsource service to any of the local/international CROs or SMOs
Type of approval
CTIL/CTX and MREC applications can be processed in parallel.
Clinical trial approval process
Before initiating any clinical trial in Malaysia, the sponsor needs to submit two applications for approval, namely
- Application to the relevant IRB/IEC, and
- An application to the DCA, the executive body under the NPRA for the Import License or Exemption.
IRB/IEC approval process
The committees to whom the application should be submitted would depend on the clinical trial site.
- Government health facilities under the MOH
- According to the NIH Guidelines, all clinical trials involving MOH facilities must register with the NMRR and obtain prior approval from the MOH.
- After submission NMRR will review the documents and, if satisfied will forward them to the Medical Research and Ethics Committee (MREC) for their review and approval.
- Universities or private institutions
- Applications are to be submitted to the respective IRB/IEC of the university or institution, which will review and approve the trial proposal as per the functions of the MREC.
The application for IRB/IEC approval can be made by the principle investigator responsible for the conduct of the trial.
The list of documents for IRB/IEC include
- Trial protocol
- Informed consent form
- Consent form updates
- Subject recruitment procedures and other written information to be provided to subjects
- Investigator’s Brochure.
Import License or Exemption
- The applicant needs to submit the applications following the guidelines for CTIL/CTX to the NPRA.
- The NPRA screens the application dossier for completeness before handing it over to the DCA.
- PI/CRO/sponsor, who intends to submit to MREC, shall register on the National Medical Research Register (NMRR) website and get a user account. Then onwards, applicants can use this account for all submissions.
- CTIL/CTX application is processed in 30 working days, while the MREC approval process takes 50 working days (if no amendments to the submitted documents are required).
- The sponsors can submit both CTIL/CTX and MREC applications in parallel.
- If the DCA and MREC grant approval (or other accepted IRB/IEC), then the NPRA issues the CTIL/CTX and regulatory approval letters to begin the clinical trial.
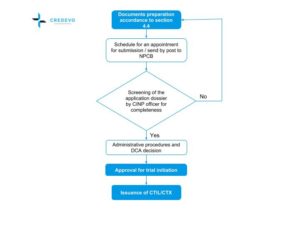
NPCB: National Pharmaceutical Control Bureau, CTIL: Clinical trial import license, CTX: clinical trial exemption, DCA: Drug Control Authority, CINP: Centre for Investigational New Product
Timeline for clinical trial approval
The average timeline for regulatory and IRB approval is about 3-4 months.
Do you require support for conducting clinical trials in Malaysia, or do you have any questions?
We’d love to help you conduct clinical trials in Malaysia. Connect with experienced and resourceful sites, service providers, and experts in Malaysia. Provide your details below.
Looking for more Southeast Asian regions for your Clinical Trials, follow the links below
- Clinical Trials in Indonesia
- Conducting clinical trials in Vietnam – why and how to start?
- Philippines – Clinical Trial Regulatory Process
- Singapore Clinical Trial Regulatory Process
- Thailand’s Clinical Trial Regulatory Scenario – Simplified (Part – 1, Part – 2).
2 thoughts on “Malaysia – Why and How to Start Your Clinical Trials?”
Comments are closed.