Acute Myeloid Leukemia – Phase I/II Clinical Trial Feasibility
Acute myeloid leukemia (AML) is a cancer of the myeloid line of blood cells, characterized by the rapid growth of abnormal cells that build up in the bone marrow and blood that interfere with normal blood cells. It’s sometimes called acute myelocytic, myelogenous, or granulocytic leukemia.
Causes of AML
The causes of AML remain largely unknown, but it is thought to result from damage to one or more of the genes that normally control blood cell development.
Certain factors have been identified which may put some people at an increased risk include
- Very high doses of radiation (either accidentally or therapeutically).
- Exposure to industrial chemicals (like benzene).
- Certain types of chemotherapy used to treat other cancers.
- Some congenital disorders associated with the development of AML, like Down’s syndrome, Bloom syndrome, and Fanconi’s anemia.
- Some people with pre-existing blood disorders including certain myelodysplastic syndromes (MDS) and myelofibrosis, aplastic anemia, and paroxysmal nocturnal.
- Haemoglobinuria has a higher risk of developing AML.
- Cancer-causing substances in tobacco smoke.
AML Phase 1 and Phase 2 Clinical Trial Feasibility was conducted on CREDEVO. This was an interventional, randomized, open-label, single-arm study with multiple ascending doses. The feasibility was run in medical centers and sites located in Canada.
AML prevalence
- The most common types of leukemia in adults are AML and CLL (Chronic Lymphocytic Leukemia).
- In 2017, 1,315 Canadians were diagnosed with acute myelogenous leukemia and 1,047 Canadians died from acute myelogenous leukemia.
- The incidence of AML increases with age, the median age at diagnosis is 67 years. AML accounts for about 90% of all acute leukemias in adults but is rare in children.
- There are approximately 10,500 new cases each year in the United States and accounts for 1.2% of all cancer deaths in the United States.
- Estimated global AML new cases in 2018 will be around 19,520 which is 1.1 % of all new cancer cases and estimated deaths in 2018 maybe around 10,670, which is 1.8 % of all cancer deaths.
Symptoms of AML
- Lethargy, fatigue, and /or Fever
- Pale skin
- Shortness of breath, easy bruising, and bleeding
- Frequent infections,
- Bone pain
Occasionally spread may occur to the brain, skin, or gums. AML progresses rapidly and is typically fatal within weeks or months if left untreated.
AML classification
Determining the stage (extent) of the cancer is very important to treat AML. The stage is based on the size of the tumor and how far the cancer has spread.
Two of the main systems that have been used to classify AML into subtypes are the
French-American-British (FAB) classification system.
AML is classified into eight different subtypes based on the examination of the leukemic cells under the microscope. The type of blood cell involved and the point at which it stopped maturing properly in the bone marrow is classified into subtypes M0 through to M7.
World Health Organisation’s classification system
The current World Health Organisation’s classification system for AML uses additional information obtained from more specialized laboratory techniques like genetic studies to classify AML more precisely.
- Acute myeloid leukemia with recurrent genetic abnormalities
- AML with myelodysplasia-related changes
- Therapy-related myeloid neoplasms
- Myeloid sarcoma
- Myeloid proliferations related to Down syndrome
- Blastic plasmacytoid dendritic cell neoplasm
- AML not otherwise categorized
Diagnosis of AML
The first step in the diagnosis is Full blood count (FBC) or Complete blood count (CBC).
Bone marrow examination
Involves bone marrow aspiration and biopsy
- A bone marrow aspiration removes a sample of the fluid with a needle.
- Bone marrow biopsy is the sample of bone marrow, taken usually from the back of the pelvic (hip) bone to examine the number and type of cells present and the amount of hemopoiesis (blood-forming) activity taking place.
Molecular and genetic testing
- Examining the genes in the leukemia cells is important as AML can be caused by a buildup of mistakes (also called mutations) in the cell’s genes. Identifying these mistakes helps diagnose the specific subtype of AML and choose treatment options.
- The results of these tests can also be used to monitor how well treatment is working. More common molecular or genetic tests used for AML are Cytochemical and immunohistochemical tests.
Lumbar puncture also called a spinal tap
A sample of cerebrospinal fluid (CSF) is taken to find out if it contains leukemia cells or blood.
AML Phase 1 and Phase 2 Clinical trial feasibility was assessed with sites/investigators from Canada. Feedback from sites/investigators has been discussed below.
Treatment of AML
Chemotherapy is the main form of treatment for AML is divided into two phases: Induction and Postremission (or consolidation) therapy.
The initial aim of treatment is to destroy leukemic cells (no evidence of leukemic cells in the blood and bone marrow and remission (normal blood cell production and normal blood counts are restored).
Induction
- All FAB subtypes except M3 are usually given induction chemotherapy with cytarabine and an anthracycline (most often daunorubicin). Up to 70% of people with AML will achieve a remission with this protocol.
- The M3 subtype of AML, also known as acute promyelocytic leukemia (APL), is almost universally treated with the drug all-trans-retinoic acid (ATRA)
Consolidation
- Even after complete remission is achieved, leukemic cells likely remain in numbers too small to be detected with current diagnostic techniques and almost all the people with AML will eventually relapse.
- The type of post-remission treatment used will depend on several factors including the type of disease involved, how well it responded to induction therapy, age, and general health.
- People will typically undergo an additional three to five courses of intensive chemotherapy (postremission or consolidation therapy).
Relapsed AML
For people with relapsed AML, the only proven potentially curative therapy is a hematopoietic stem cell transplant if one has not already been performed. [1][2]
Prognosis
- The genetic makeup of the leukemic cells is the most important factor in predicting the prognosis in AML which depend on factors such as
- Medical history
- Type and stage of cancer
- Characteristics of the cancer
- Treatments are chosen and response to treatment
- Usually, those younger than 60 years of age, have a more favorable prognosis than older adults.
- Older people may also have other health conditions that make it difficult for them to cope with the side effects of treatments for AML.
- An early relapse means, leukemia returns soon after treatment and is linked with a less favorable prognosis.
- A serious, uncontrolled infection at the time of diagnosis is a less favorable prognostic factor.
Clinical trials on AML
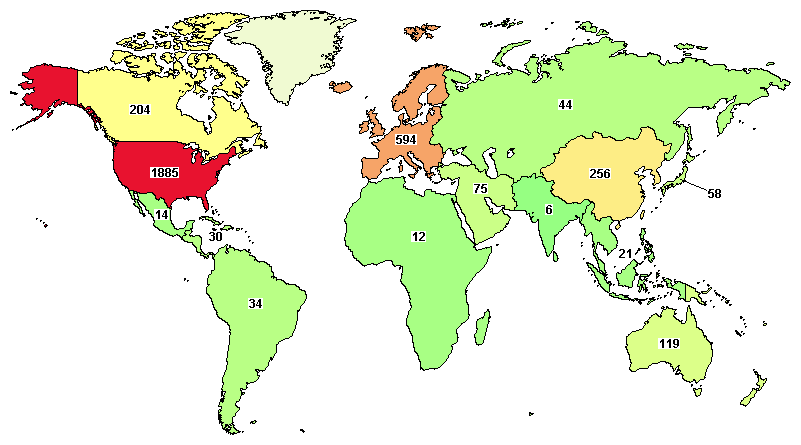
Total clinical trials going around the globe on AML are 2,776, where major trials are going in the United States (1,885), Germany (239), Canada (204), France (244), and Italy (156).
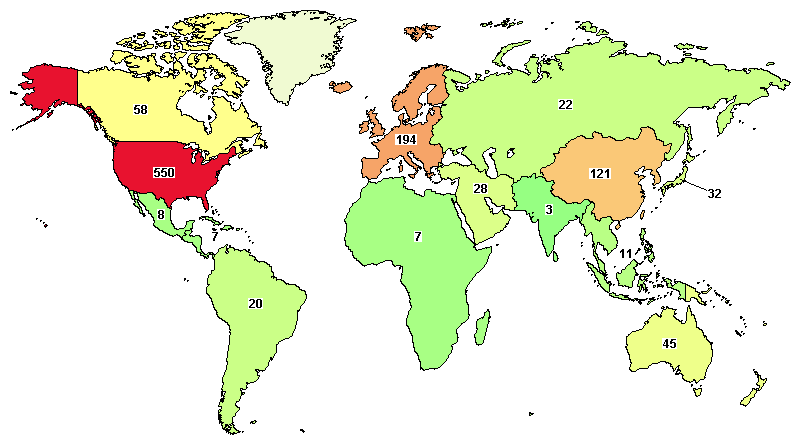
The total ongoing trials on AML are 785, where major clinical studies are in the United States (550), France (90), Germany (77), China (75), and Canada (58).
[Updated on 20-Jul-20]
AML – Clinical Trial Feasibility on CREDEVO
This Phase 1 and Phase 2 clinical study was an interventional, randomized, open-label, single-arm, with multiple ascending doses.
The feasibility was run in medical centers and sites located in Canada.
Subjects diagnosed with AML, with age greater than or equal to 18 years, were included in the study.
Patients for intensive chemotherapy as first-line, second-line, or third-line of treatment were excluded from the study.
Feasibility results
- Clinical trial feasibility was assessed with sites/investigators from Canada.
- Feedback from sites/investigators was 5 patients / 2 months.
Contact Investigators From This Feasibility Assessment
Would you like to contact these investigators and assess feasibility with them for your clinical trial?
Just provide your email below and further details will be sent to you. Don’t worry, your email will not be published or shared.
For other details, click here to contact us.
References
- https://en.wikipedia.org/wiki/Acute_myeloid_leukemia#Diagnosis
- https://www.leukaemia.org.au/disease-information/leukaemias/acute-myeloid-leukaemia/how-is-it-diagnosed/
- http://www.cancer.ca/en/cancer-information/cancer-type/leukemia-acute-myelogenous-aml/prognosis-and-survival/?region=on
- https://www.cancer.net/cancer-types/leukemia-acute-myeloid-aml/diagnosis
- https://seer.cancer.gov/statfacts/html/amyl.html
- https://clinicaltrials.gov/ct2/results/map?recrs=ad&cond=Acute+Myeloid+Leukemia&map=
One thought on “Acute Myeloid Leukemia – Phase I/II Clinical Trial Feasibility”
Comments are closed.